Early on in my Hashimoto’s journey, I experienced a lot of digestive symptoms and discomfort, most of which I didn’t initially link to hypothyroidism or my autoimmune condition.
What I didn’t understand at the time was how closely gut health is tied to thyroid and immune system health!
The gut microbiome describes the trillions of bacteria and the substances they produce, found primarily in the large intestine.
Among its many responsibilities in the body, the gut microbiome supports the gut’s protective mucus barrier lining. This barrier allows vital nutrients from our digested food to be absorbed into the bloodstream, while simultaneously blocking the entry of bacteria and toxins. This is called “selective permeability,” and it essentially means that the gut “knows” which molecules to let pass through based on size.
When things go awry, this mucus barrier can break down, increasing intestinal permeability and resulting in “leaky gut.” Partially digested food, bacteria, and other toxins are then able to pass through the damaged barrier and are freely absorbed into the bloodstream, activating the immune system.
Researchers have connected intestinal permeability to every case of autoimmune disease, including Hashimoto’s. [1] Alessio Fasano’s research has found that in order for autoimmune disease to develop, there must be three factors present – a genetic predisposition, intestinal permeability, and an environmental trigger (such as an infection or major stressor).
Along with nutrient deficiencies and reactive foods (such as gluten), the most common triggers for intestinal permeability that I see in my clinical practice include intestinal infections like Blasto, H. pylori, or small intestinal bacterial overgrowth (SIBO), stress, digestive enzyme deficiencies, and an imbalance of gut bacteria, often called gut dysbiosis (the focus of today’s article).
Gut dysbiosis has been getting a lot of attention lately, and I’m excited to share some new research and protocols with you!
Read on to find out about:
- The thyroid and autoimmunity connection to dysbiosis and leaky gut
- Common causes of gut bacteria imbalances
- The role of the various metabolites the gut bacteria produce
- Diagnosing gut dysbiosis and leaky gut
- How to address gut dysbiosis
The Thyroid and Autoimmunity Connection to Dysbiosis and Leaky Gut
When it comes to the connection between dysbiosis, leaky gut, thyroid dysfunction, and autoimmunity, here are some key points to keep in mind:
- Some increased level of intestinal permeability (leading to leaky gut) is seen in all autoimmune conditions, including Hashimoto’s.
- The health and composition of the gut microbiome are influenced by thyroid hormones, and vice versa.
- The active thyroid hormone, triiodothyronine (T3), is the most important regulator of the development of epithelial cells of the intestinal barrier.
- Thyroid functionality is influenced both directly and indirectly by the balance of bacteria in the gut.
The graphic below demonstrates the reciprocal relationship between the thyroid and dysbiosis, and the consequences when there are imbalances in either.
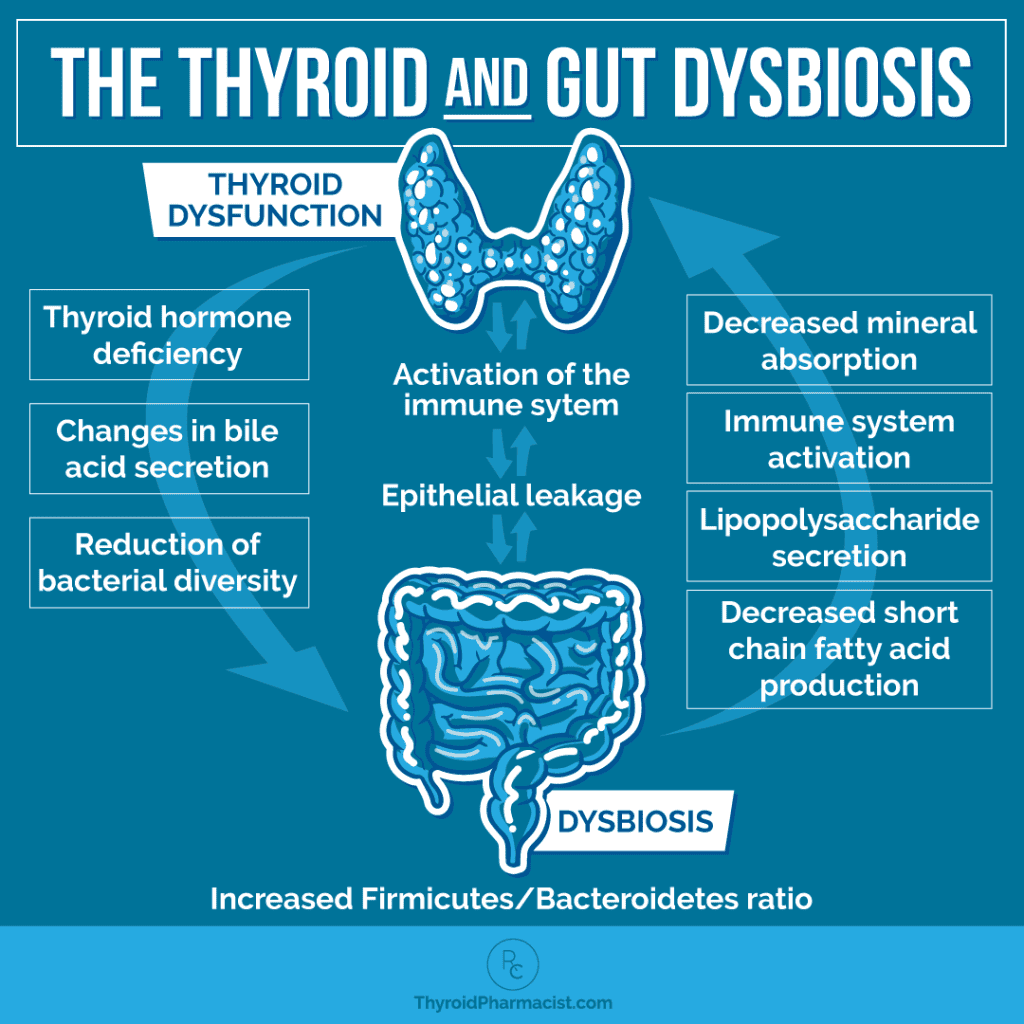
A sluggish thyroid reduces gut motility, which can result in a greater number of pathogenic bacteria and a greater risk for gut infections like SIBO. Hypothyroidism can cause a decrease in bile secretion, affecting digestion and nutrient absorption (which in turn can further affect thyroid function).
The downstream effects of poor thyroid function result in a reduction of bacterial diversity and abundance.
Dysbiosis leads to leaky gut, which we know to be one of three conditions that must be present for a person to develop autoimmune disease.
Dysbiosis Patterns and Hypothyroidism
Studies have shown that those with hypothyroidism have an altered composition of gut bacteria with reduced populations of the beneficial short-chain fatty acid (SCFA)-producing bacteria, and higher levels of inflammation-driving lipopolysaccharides (LPS)-producing bacteria.
One 2020 study involved 52 hypothyroidism patients and 40 healthy controls. Their gut microbiota were analyzed by newer 16SrRNA sequencing technology, and it was found that there were significant differences in the gut bacteria between the groups. Researchers found that levels of four specific bacteria (Veillonella, Neisseria, Paraprevotella, and Rheinheimera) could actually identify whether a subject had hypothyroidism or was from the healthy control group! Additionally, the SCFA-producing ability of those with hypothyroidism was significantly decreased, and LPS levels increased. [2]
Levels of bacteria found in the hypothyroidism group were correlated with levels of thyroid hormones, including T3, T4, and TSH.
The researchers also performed fecal microbiota transplantation (FMT), taking flora from both human groups and transplanting them into mice. The results were quite interesting, as the mice receiving the bacterial transplant from the hypothyroidism group displayed decreased total thyroid hormone levels after the transplant!
Dysbiosis and Nutrient Uptake
Iodine, selenium, iron, and zinc are important thyroid-supportive nutrients, and the composition of the gut bacteria affects how these nutrients are absorbed. Thyroid dysfunction itself can modify gut microbiome composition, as well as impact the absorption of these minerals. [3] Iodine and iron are vital to thyroid hormone synthesis, while selenium and zinc are important in T4 to T3 conversion and immune health. [4] Click through to the linked articles to learn more about these nutrients.
Thyroid Hormone Medication Uptake and the Microbiome
I’ve previously written about how SIBO is common in hypothyroidism (affecting 50 percent of people with hypothyroidism in one study), and research has found that when such an overgrowth occurs, it results in changes to microbiome composition and medication absorption, and higher doses of thyroid medication may be required.
The same result has been found for the common thyroid-triggering infection H. pylori as well.
Interestingly, a newer study suggests that beneficial bacteria can also impact thyroid hormone doses. One study found that the application of several probiotics (three Bifidobacterium spp., four Lactobacillus spp., and Streptococcus thermophiles) reduced the amount of levothyroxine dose adjustments compared to a control group without probiotics. [5] Another study showed that Lactobacillus reuteri can increase thyroid hormones. [6]
Dysbiosis Sets the Stage for Hashimoto’s
We already talked about how dysbiosis is a potential cause of intestinal permeability, which we know is one of the three factors required for Hashimoto’s to develop. Let’s dive into the new research regarding what gut bacteria are out of balance in Hashimoto’s and what we can do about it.
One 2017 study found that Hashimoto’s patients had dramatically different bacterial makeup (diversity and abundance) compared to healthy controls. One example is the Firmicutes/Bacteroidetes ratio, which has been found to be significantly higher in those with Hashimoto’s and other diseases such as IBS. Higher ratios are generally a sign of ill health and are associated with microbial imbalance. A higher ratio of Firmicutes to Bacteroidetes is also linked to obesity, and is even considered a hallmark of obesity in scientific literature. [7] After weight loss, this ratio improves.
In the 2017 study, those with Hashimoto’s were found to have lower levels of the bacteria that are crucial for intestinal barrier integrity and modulating inflammation. A few examples are Bacteroides which produce SCFAs, Faecalibacterium which produce butyrate and are anti-inflammatory, and Prevotella, which are known to produce anti-inflammatory metabolites. [8]
Most interesting in this study was that there were correlations between higher levels of certain bacteria and the presence of thyroid antibodies. These bacteria include Blautia, Roseburia, Ruminococcus torques, Romboutsia, Dorea, Fusicatenibacter, and Eubacterium hallii.
Dysbiosis, Thyroid Nodules and Thyroid Cancer
Dysbiosis has been found to be connected to both thyroid nodules and thyroid cancer.
In one study, the gut composition in participants with high-grade (high malignancy risk) thyroid nodules was characterized by lower butyrate production, decreased overall bacterial diversity, and alterations in the overall microbial composition (fewer butyrate producers). Researchers highlighted the potential for specific gut microbiota to be identified as a potential therapeutic target to help regulate thyroid metabolism. [9]
Dysbiosis has also been reported in thyroid carcinoma, in which an increased number of carcinogenic and inflammatory bacterial strains were observed. [10]
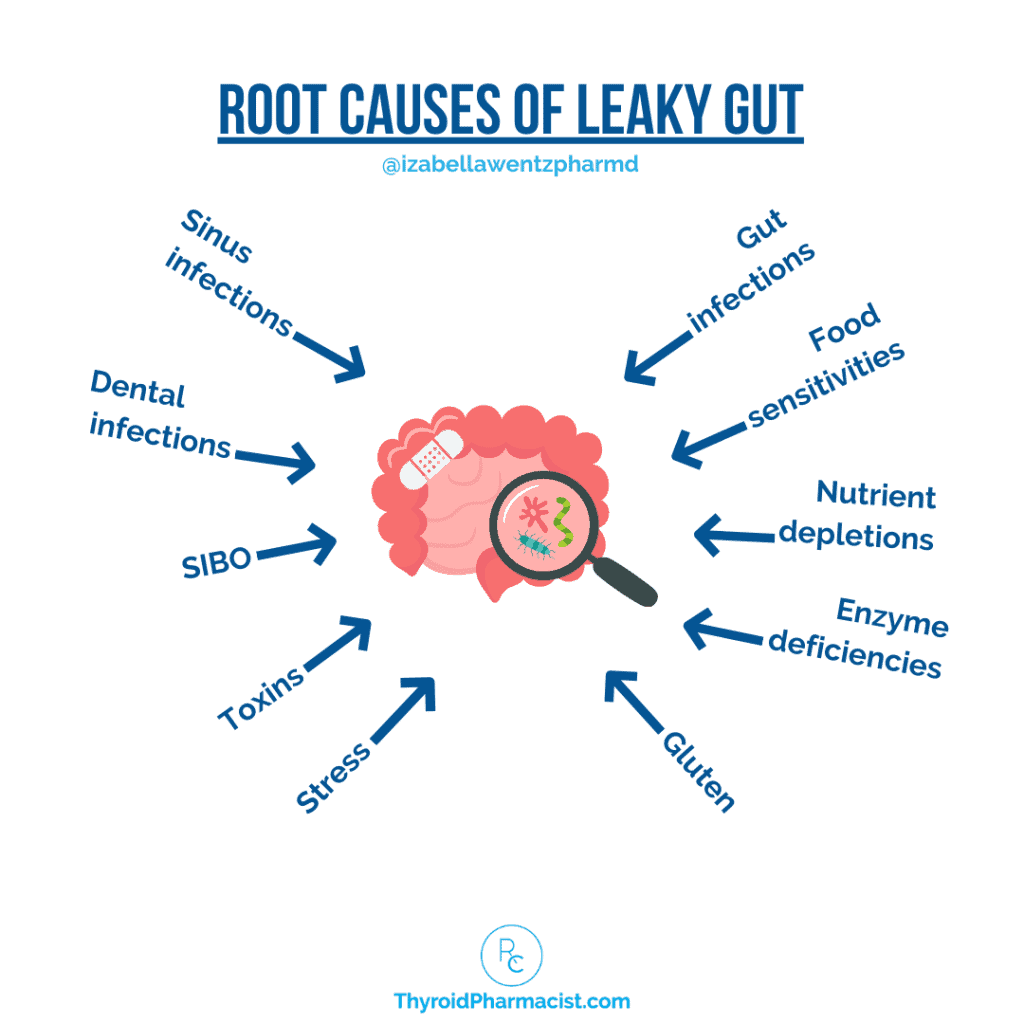
Common Causes of Gut Bacteria Imbalances and Leaky Gut
Numerous factors affect the composition of the microbiota and levels of intestinal permeability, and it isn’t a coincidence that many of these are also common triggers for Hashimoto’s.
- Genetic predisposition – Certain genetic variants can influence the body’s recognition and response to bacteria, and can lead to changes in the microbiome. [11]
- Maternal colonization of microbiota during birth – When a baby passes through the vaginal canal, they are inoculated with mom’s bacteria from her vaginal microbiome (those born via Cesarean have lower bacterial diversity). [12]
- Diet – What we eat can influence the makeup of our microbiome and the gut’s level of permeability. For example, certain food additives can alter bacteria in the gut. [13] Gluten in particular has been shown to cause increased intestinal permeability in those with a sensitivity. [14]
- Food sensitivities – Eating foods we’re sensitive to can contribute to inflammation in the gut, which can lead to altered intestinal permeability. This can affect the bacteria in the microbiome.
- Toxins – A number of different toxins can influence intestinal permeability and levels of various bacteria in the gut. This includes environmental toxins like pollution, as well as endogenous toxins (such as LPS). Toxic overload leading to liver dysfunction is also a common Hashimoto’s trigger.
- Stress – Studies have found that social stress, for example, decreases the level of microbes with anti-inflammatory activity, and contributes to a higher level of inflammation. [15] Chronic stress can also contribute to increased intestinal permeability. [16]
- Gut infections and parasites – Infections such as H. pylori, SIBO, Blastocystis hominis, Giardia, and Candida can cause gut bacteria imbalances. For example, an H. pylori infection (as well as the antibiotics used to treat it) can result in a reduction in bacteria diversity. [17] In addition, proton pump inhibitors (PPIs), which are usually administered together with antibiotics for H. pylori eradication, contribute further to bacterial shifts in the intestine. [18]
- Bariatric surgery [19]– Recent research has found that this weight loss surgery has a profound impact on bacteria in the gut (though often in a beneficial way). [20]
- Antibiotics – Broad-spectrum antibiotics such as amoxicillin, tetracycline, and fluoroquinolone are the worst offenders, decreasing the diversity and abundance of Firmicutes and Bacteroidetes. [21]
- Drugs – Common medications such as proton pump inhibitors and birth control pills (which are thyroid-toxic as well) can affect the composition of the microbiota. [22]
- Nutrient deficiencies – Modern farming practices (which often result in fewer nutrients in soils and foods) are partly to blame for nutrient deficiencies, but low digestive enzymes and low stomach acid may also be factors. [23] For instance, low levels of glutamine and zinc, two substances required for rebuilding the intestinal lining, can lead to intestinal permeability.
- Lifestyle – Exercise, sleep, alcohol, and nicotine have all been shown in the research to alter the microbiota composition and affect the permeability of the gut lining. [24]
- Hormones – Sex hormones have a significant effect on microbiota composition and abundance. Females and males actually have distinct microbial profiles. Estrogen dominance can be a trigger for Hashimoto’s. [25]
- Other disease states – Other disease states, especially metabolic/energy-related conditions such as obesity, can be factors in microbiome imbalances. Animal studies suggest that obesity (caused by either genetic or environmental factors) increases populations of bacteria that produce the endotoxin lipopolysaccharide (LPS), and decreases LPS-suppressing bacteria. Obesity may also impair gut barrier integrity. [26]
- Infections – Underlying infections like sinus infections or dental infections can cause low-grade stimulation of the immune system and contribute to the development of leaky gut. [27] Infections can also be a trigger for Hashimoto’s. [28]
- Aging – Changes typically seen in the microbiome of older individuals are attributed to diet, altered lifestyles, reduced mobility, reduced intestinal capability (reduced stomach acid, etc.), decreased immune function and disease states, the use of medications, and recurrent infections. [29]
Benefits of Beneficial Bacteria and Risks of Inflammatory Metabolites
Gut bacteria produce metabolites by breaking down food, chemicals, drugs, or even the gut’s own tissue; these metabolites can exert beneficial or detrimental effects.
In dysbiosis, fewer bacteria are available that produce beneficial metabolites, and there may be an increased number of potentially harmful metabolites.
Let’s talk for a moment about just a few of these important metabolites, both good and bad.
Short-Chain Fatty Acids (SCFAs) – Good Guys!
When we eat certain foods, there are bacteria in the intestines that break down otherwise indigestible fibers into compounds called short-chain fatty acids (SCFAs), which are known for their beneficial health effects.
The three most abundant SCFAs include butyrate, acetate, and propionate. Valerate, formate, caproate, and others make up the remaining SCFAs.
Fibers from fruits and vegetables like apples, oranges, apricots, carrots, bananas (green is best), garlic, onions, asparagus, artichokes, leeks, beans, legumes, rice, grains (wheat bran, oat bran, rye, barley), and guar gum, are fermented by gut bacteria and produce SCFAs.
SCFA production is impacted by microbiota composition (only specific beneficial gut bacteria are involved in producing SCFAs), the diet (prebiotics/fibers), and gut transit time. [30] Some SCFA-producing bacteria include Lactobacillus, Bifidobacterium, Bacteroides spp., Faecalibacterium, Roseburia spp., Ruminococcus, Clostridium leptum, Eubacterium, Propionibacterium, Akkermansia, and Coprococcus spp. [31]
It’s important to note that in hypothyroidism, gut motility is often reduced (many of my clients initially experience constipation), and a slower transit time has been shown to result in lower SCFA concentrations overall, as well as lower numbers of butyrate-producing bacteria, and a reduced percentage of butyrate. We’ll talk in a moment about why butyrate in particular is so important. [32]
Research has confirmed that reduced levels of SCFA-producing bacteria and SCFA metabolites are found in a variety of conditions, including type 2 diabetes, IBD, celiac disease, hypothyroidism, and others. [33]
While all of the SCFAs are beneficial to gut health, there is one in particular that I want to hone in on, as it has been shown to be particularly beneficial, and I’ve found it can help correct dysbiosis – and that is butyrate.
Butyrate provides a number of benefits:
- It protects the intestinal barrier. Butyrate is important for the health of the gut lining. It supports the integrity of the intestinal lining by regulating the tight junctions (it does this together with T3 thyroid hormones, so someone with hypothyroidism may have a more permeable gut given the lack of active T3), and by supporting intestinal mucus production. [34] Butyrate also controls intestinal cell repair. Butyrate fuels the intestinal cells – providing about 70 percent of their energy – and enables the intestines to regrow new cells at a very fast pace. [35]
- It controls inflammation, limits auto-reactive immune responses, and is protective of colon health. Butyrate can induce regulatory T-cells (Treg) cells and thus modulate the progression of inflammatory diseases and autoimmunity. [36] Higher levels of butyrate are positively associated with increased numbers of Tregs, while simultaneously leading to reduced concentrations of proinflammatory Th-17 cells, meaning that it helps to modulate inflammation in two ways. [37] Butyrate inhibits inflammation induced by lipopolysaccharides (LPS). [38] This, along with its ability to lower the gut’s pH level, improve gut motility, and inhibit colibactin (implicated in colon cancer), is supportive of colon health. [39]
- It is beneficial to brain health. One recent study found that colonizing germ-free mice with butyrate-producing bacteria restored blood-brain barrier permeability. [40] Butyrate-producing bacteria appear to mitigate psychological stress in human subjects, as well as reduce anxiety in rats. [41] In another study, children who followed a high-fiber diet had better cognitive control (memory, multitasking) than those who ate a lower-fiber diet. [42] Thus, natural dietary sources of butyrate, such as resistant starches, appear to be an easy and low-risk intervention to address many neurological issues.
- Insulin control: Butyrate has been shown to enhance fatty acid oxidation, reduce fat production, increase insulin sensitivity, and improve mitochondrial function. One study showed that dietary supplementation of butyrate can prevent and treat diet-induced obesity and insulin resistance in mouse models. [43] Although there are numerous studies suggesting the effect of butyrate on alleviating diet-induced obesity and insulin resistance (in mice and in humans), a few studies have found an opposite effect. [44]
The main butyrate-producing bacteria belong to the phylum Firmicutes, in particular Clostridium leptum, Faecalibacterium prausnitzii, Eubacterium hallii, Anaerostipes spp., Eubacterium rectale, Coprococcus catus, Coprococcus comes, Coprococcus eutactus, and Roseburia spp. [45]
Additionally, butyrate can be created from the fermentation process of certain amino acids such as lysine, serine, histidine, methionine, and glutamate. [46] Some types of butter, cheese, and cow’s milk contain small amounts of butyrate as well.
Hemorrhoids: A very strange predictor of Hashimoto’s!
A 2020 study of 6,486 patients with hemorrhoids and 25,944 patients without, found that the risk of developing Hashimoto’s was 2.06 times higher in the hemorrhoid group.
If you look at common risk factors (between having hemorrhoids as well as Hashimoto’s), you’ll see that they do share several things in common. Typically there is constipation (in hypothyroidism due to slower gut motility and bile changes), a diet low in fiber (lower SCFAs), and the obesity link (found to be associated with lower SCFA production in hypothyroidism), which is also a risk factor for hemorrhoids due to increased intra-abdominal pressure during bowel movements (an interesting predictor!). [47]
Lipopolysaccharides (LPS) – Bad Guys!
LPS are located in the outer cell membrane of certain gram-negative bacteria such as E. coli, and can act as endotoxins. They trigger inflammation and increase gut permeability. They are also associated with liver damage, neurological conditions, and diabetes. [48] Gut dysbiosis promotes the production of LPS. [49]
The presence of LPS induces Toll-like receptor 4 (TLR4) activation. TLR4 stimulates pro-inflammatory cytokines and further reduces the integrity of the gut barrier. Prolonged activation of TLR4 has been linked with a number of hereditary and neurodegenerative conditions, as well as with cancer and autoimmune diseases. [50]
A more recent study shows that LPS can downregulate pituitary and thyroid function, decreasing thyroxine (T4) and TSH secretion. [51]
Interestingly, anatabine, a now off-market supplement that has been shown to reduce thyroid antibodies, claimed its mechanism of action was through neutralizing LPS.
Diagnosing Gut Dysbiosis and Leaky Gut
So how do you know if you have an imbalanced gut microbiome and leaky gut – and more importantly, what can you do about it?
Gastrointestinal symptoms such as constipation, diarrhea, bloating, and pain are good indicators that something may be out of balance, but in my experience, only about half of people seem to experience these symptoms.
Conventional practitioners are unlikely to consider imbalances in the gut microbiome and/or leaky gut when it comes to making a formal diagnosis – although these days, it seems that they are more likely to recommend over-the-counter probiotics when prescribing antibiotics than they used to. The issue here is that often, the probiotic doesn’t include a diverse enough level of beneficial bacteria at a high enough dose!
This is why I recommend alternative routes to test for dysbiosis. I like to use the Gut Zoomer test, a comprehensive stool test that analyzes over 300 microorganisms in your large intestine and can help identify imbalances in your gut microbiome. It’s one of the only tests that also reports on short-chain fatty acids so that you can tell which ones may be deficient.
It also tests for infections and parasites, and I was intrigued by this test when a colleague shared a few years ago that it revealed an exotic parasitic infection in an individual with mysterious symptoms.
You can order the Gut Zoomer test here (you will either need a provider to order it for you, or they can help you find one).
Another option is the GI-MAP (GI Microbial Assay Plus) – Diagnostic Solutions Kit. This test checks for bacteria, fungi, parasites, and viruses in the gut. This test reports on a number of key markers of digestion, inflammation, and immune function. It also measures the bacterial balance of normal flora and digestive markers, like elastase and secretory IgA, which provide further insight into overall digestive and intestinal health. It doesn’t get into detail about your SCFA breakdown, but it does report on beneficial vs. pathogenic bacteria levels. It also covers many of the commonly seen gut pathogens that I come across with my Hashimoto’s clients, including H. pylori and the parasite Blastocystis hominis (Blasto).
As much as I love this test, it doesn’t catch every infection, and in some cases, I may recommend ordering more than one gut test to rule out more infections. In general, the more tests you do, the more likely you are to identify an infection.
Self-order options for the GI-MAP test include:
Addressing Gut Dysbiosis
As mentioned, recognition and treatment of gut dysbiosis and leaky gut are limited in conventional medicine. Without testing, you may not know you have gut dysbiosis, but if you have Hashimoto’s, you know that you have some degree of intestinal permeability, and will likely benefit from interventions meant to support the gut barrier and microbial balance.
In the last few years, I created the Gut Recovery Program, which focuses on solving complex gut issues I commonly see in autoimmune patients. One of the protocols in the program is focused on addressing dysbiosis and low butyrate levels.
This is a general protocol I like to use for gut dysbiosis, and I have found it to be very effective and helpful for most people. It includes the use of probiotics, as well as three additional microbiome stars that can make a big impact on gut health and short-chain fatty acids:
Probiotics
To address dysbiosis, we want to start shifting the microbiome in a positive direction by increasing levels of beneficial bacteria, and decreasing levels of pathogenic bacteria. I’ve spoken about probiotics at length, as they can be so helpful for people with Hashimoto’s.
Many years ago, I thought that taking probiotics from the drugstore or eating yogurt would be enough to balance gut microbes, but then I began to learn about how different strains of probiotics have different benefits, and how the dose of the same strain can turn a “maintenance probiotic” into a “therapeutic probiotic.” I also learned (the hard way), that taking the wrong type or dose of probiotics can be disastrous!
People with autoimmunity tend to have lower amounts of the probiotic bacteria Lactobacillus and Bifidus, and higher amounts of the opportunistic E. coli and Proteus bacteria. [52]
I’ve seen this pattern of low levels of probiotic bacteria with high levels of opportunistic bacteria on my own lab tests, as well as on the tests of many clients with Hashimoto’s who have had stool testing to quantify microbial flora.
In order to get the high therapeutic levels of probiotics that are often required to see a shift in bacteria levels, it’s necessary to use supplements. I’ve written an article about the best types of probiotics for Hashimoto’s that covers which types can be most helpful, which ones to avoid, guidance and dosing, and information on fermented foods, which contain helpful probiotics.
Berberine
I first learned about berberine a decade ago, while attending functional medicine conferences to learn more about how to heal my own Hashimoto’s. I remember speaking to a few doctors who mentioned that berberine seemed to help their Hashimoto’s patients feel better and reduce thyroid antibodies. I have not seen this in the published research, but I have seen it clinically! Berberine has various benefits, including metabolic and blood sugar balancing benefits, and has even been shown to reduce intestinal permeability. [53] I also wonder if this improvement is due in part to the antimicrobial activity. It can also support a healthy weight by minimizing carbohydrate absorption. [54]
Berberine contains compounds called alkaloids that have broad-spectrum antimicrobial and antiparasitic properties, making it a wonderful ally for eradicating gut infections and supporting healthy microbial balance (thus potentially helping Hashimoto’s as well). [55]
In certain cases of Blasto infections, I pair berberine with oil of oregano and S. boulardii. Its antifungal properties also make it an effective treatment for Candida. [56] It has also been shown to be an effective treatment for H. pylori. Furthermore, it has been found to help clear out pathogenic bacteria overgrowths such as Strep and Staph, as well as protozoa such as Dientamoeba, Giardia, and Pentatrichomonas. [57]
As a bonus, it’s been shown to increase levels of bacteria, such as Blautia and Allobaculum, which produce SCFAs, resulting in higher levels of SCFAs in the gut. [58]
In addition to its impact on gut infections, berberine also has blood sugar-balancing properties (blood sugar issues are so common in Hashimoto’s).
Because I’ve found this supplement to be so helpful, I developed a Berberine formulation as part of my Rootcology supplement line, which contains 400 mg of berberine. Other high-quality options include Berberine 500 by Vital Nutrients and Berberine UltraSorb by Pure Encapsulations.
In addition, I’ve recently learned about two superfuels for beneficial bacteria that can create a really positive impact on the microbiome: resistant starch and butyrate.
Resistant Starch
As the name implies, resistant starch (RS) is a type of carbohydrate that resists digestion. It’s not digested in the small intestine and moves on to the large intestine, where it’s fermented. These fermented fibers act as a prebiotic – food for the good bacteria in your gut – and can increase levels of SCFAs, which are used as fuel for both beneficial microbes and the cells of the intestinal lining.
You can find RS in foods like plantains, green bananas, chickpeas, Jerusalem artichokes, and lentils. Cooked and cooled potatoes, as well as rice, also contain high amounts of RS.
I love resistant starch because of its many benefits:
- It feeds the good bacteria. When resistant starch reaches our large intestine (colon) unchanged and undigested, it feeds the friendly bacteria that live there. As such, resistant starch is a very effective prebiotic (food for probiotics). [59]
- It’s a safer prebiotic. Popular prebiotics like inulin and artichoke are high in FODMAPs and may be problematic for many people with gut issues, particularly IBS and leaky gut. Resistant starch is low in FODMAPs and is a safer option.
- It makes more butyrate. When resistant starch arrives in the colon undigested, it gets eaten and fermented by our friendly bacteria, thus producing beneficial SCFAs such as butyrate.
- It creates a less friendly environment for pathogenic bacteria. This is particularly good news for people with dysbiosis or Candida. [60]
- It helps with healthy weight management. By keeping us feeling full longer, it may help us manage our weight more efficiently. [61] It also lowers blood sugar and improves insulin sensitivity, which can support a healthy weight as well. Because resistant starch passes through our GI tract undigested, it does not spike our blood glucose or insulin. [62]
- It helps with carb concerns. For those who typically restrict their carbs, know that resistant starch is absorbed in the large intestine and is a slow-burning carb. It provides seven to nine hours of stable blood sugar, so it’s a good carb (similar to green leafy vegetables).
While we can get RS from the foods mentioned above, I also love using supplemental RS to ensure you’re getting enough to support the gut. Rootcology Paleo Starch is a blend of organic green banana flour and organic potato starch. Green bananas are a great source of RS, which can help good bacteria grow.
Although prebiotics can generally be taken along with probiotics, when it comes to resistant starch, I recommend taking one scoop blended in a beverage or mixed with food, at bedtime, as it can help with stabilizing blood sugar levels and with sleep quality.
Aloe vera can also be helpful, as it is loaded with enzymes, and has potent anti-inflammatory and antioxidant properties, but also has prebiotic benefits!
*Note: As with probiotics, I always suggest starting low and going slow when introducing prebiotics, as you may have increased symptoms if your gut flora changes too rapidly.
Butyrate
As discussed earlier, butyrate is one of the most well-known and important SCFAs. It can help support the integrity of the intestinal lining, healthy gut flora, and bowel function, as we rebalance the gut. [63]
Butyrate has many other benefits, including controlling inflammation, limiting autoimmune responses, supporting brain health, and even supporting a healthy weight, because it can enhance fatty acid oxidation, reduce fat production, and increase insulin sensitivity.
Butyrate has been shown to have important immunomodulatory functions, and patterns of low butyrate have been observed in autoimmunity. [64] A 2019 study found that the bacteria that produce short-chain fatty acids (SCFAs) such as butyrate, are reduced in the gut and feces of people with inflammatory bowel disease (IBD), compared to healthy subjects. [65]
Another study that examined 196 patients with thyroid nodules, found that those with high-grade thyroid nodules had a reduced number of butyrate-producing microbes in their guts, along with a decreased number of gut microbial species. [66]
One strategy for increasing butyrate levels includes focusing on a micronutrient-dense, diverse, high-fiber, and low-fat diet. (You would want to remove simple sugar, refined flour, saturated and trans fats, high-fructose corn syrup, and other processed foods.) A high-fiber diet containing a variety of different foods is associated with a protective effect regarding dysbiosis. It also increases SCFA production, lowers LPS activation and supports intestinal barrier function. [67]
Beyond shifting the microbiome to more butyrate-producing microbes, and boosting levels of butyrate by adding prebiotics and resistant starch to your diet, you can also supplement with butyrate directly. It’s a bit of a “if you build it, they will come” effect!
Butyrate can influence the overall composition of the gut microbiota. A healthy gut environment promoted by butyrate can reduce conditions that favor the proliferation of pathogenic bacteria, including those that reduce sulfur (and make us gassy). Enhanced fermentation as a result of fiber intake and butyrate production creates a gut environment where pathogenic bacteria have a difficult time surviving.
Many individuals have reported that butyrate supplements help with food sensitivities, and some research has even reported that it can reverse food allergies! Supplementing with butyrate has been shown to increase oral tolerance to food proteins, likely because of butyrate’s ability to support the growth of beneficial bacteria, and support a healthy gut lining. This helps keep undigested food proteins in the gut, rather than leaking into the bloodstream where they can trigger an allergic response. [68]
There are various options that can be taken as supplements, and I took butyrate back in 2016 with great results to resolve my egg and sulfur sensitivities. The only issue is that it completely ruined my favorite carry-on travel bag! Encapsulated butyrate has quite a distinct, stinky smell to it. Some people say it smells like rotten butter; others say it smells like cheese or nasty feet. 🙂 I didn’t mind taking it despite the smell because the health benefits were significant, and I stayed on it for quite some time when I was dealing with sulfur issues.
I had packed some butyrate capsules in a Ziploc bag in my carry-on for travel, and they spilled out from their bottle into the Ziploc bag. They made my bag smell horrific (despite still staying in the Ziploc bag), and now I no longer have my awesome pink, perfect-sized Kipling carry-on!
Being a pharmacist and formulation nerd, I was excited to create a butyrate gelcap that hides the smell of butyrate, making it more likely that you will take it, and less likely to ruin your favorite bag. 🙂
I’m excited to share that Rootcology Butyrate Balance is now available, and I hope it helps you as much as it helped me!
Takeaway
If you have gut issues, especially leaky gut, please know that there is hope!
I have natural treatment protocols in place for most of the root causes of leaky gut, and most are Hashimoto’s triggers that you can change, such as diet and lifestyle factors.
Increasing your fiber intake (and choosing the right types of fiber), adding more fermented foods to your dinner plate, and taking the right probiotics can be game-changers!
Remember that most people with dysbiosis and leaky gut will also have other root causes, such as infections, stress, or food sensitivities. Don’t forget to dig deep into your root causes!
Have you ever experienced intestinal permeability? What helped you heal it?
P.S. I love interacting with my readers on social media, and I encourage you to join my Facebook, Instagram, TikTok, and Pinterest community pages to stay on top of thyroid health updates and meet others who are following similar health journeys. For recipes, a FREE Thyroid Diet Quick Start Guide, and notifications about upcoming events, be sure to sign up for my email list!
References
[1] Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):71-78. doi:10.1007/s12016-011-8291-x
[2]Ibid.
[3] Bargiel P, Szczuko M, Stachowska L, et al. Microbiome Metabolites and Thyroid Dysfunction. J Clin Med. 2021;10(16):3609. Published 2021 Aug 16. doi:10.3390/jcm10163609
[4] Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function?. Nutrients. 2020;12(6):1769. Published 2020 Jun 12. doi:10.3390/nu12061769
[5]Ibid.
[6]Ibid.
[7] Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients?. Nutrients. 2020;12(5):1474. Published 2020 May 19. doi:10.3390/nu12051474
[8] Zhao F, Feng J, Li J, Zhao L, Liu Y, Chen H, Jin Y, Zhu B, Wei Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid. 2018 Feb;28(2):175-186. doi: 10.1089/thy.2017.0395. Epub 2018 Feb 1. PMID: 29320965.
[9] Li A, Li T, Gao X, et al. Gut Microbiome Alterations in Patients With Thyroid Nodules. Front Cell Infect Microbiol. 2021;11:643968. Published 2021 Mar 12. doi:10.3389/fcimb.2021.643968.
[10] Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients. 2020 Jun 12;12(6):1769. doi: 10.3390/nu12061769. PMID: 32545596; PMCID: PMC7353203.
[11] Nibali L, Henderson B, Sadiq ST, Donos N. Genetic dysbiosis: the role of microbial insults in chronic inflammatory diseases. J Oral Microbiol. 2014;6:10.3402/jom.v6.22962. Published 2014 Feb 25. doi:10.3402/jom.v6.22962
[12] Zhang C, Li L, Jin B, et al. The Effects of Delivery Mode on the Gut Microbiota and Health: State of Art. Front Microbiol. 2021;12:724449. Published 2021 Dec 23. doi:10.3389/fmicb.2021.724449
[13] Zhou X, Qiao K, Wu H, Zhang Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules. 2023;28(2):631. Published 2023 Jan 7. doi:10.3390/molecules28020631
[14] Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7(3):1565-1576. Published 2015 Feb 27. doi:10.3390/nu7031565
[15] Mousa WK, Chehadeh F, Husband S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front Microbiol. 2022 Feb 3;13:825338. doi: 10.3389/fmicb.2022.825338. PMID: 35185849; PMCID: PMC8851206; van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. PMID: 30066368; PMCID: PMC6187046.
[16] Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. Published 2015 Oct 14. doi:10.3389/fncel.2015.00392
[17] Martin-Nuñez GM, Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Gut Microbiota: The Missing Link Between Helicobacter pylori Infection and Metabolic Disorders? Front Endocrinol (Lausanne). 2021 Jun 17;12:639856. doi: 10.3389/fendo.2021.639856. PMID: 34220702; PMCID: PMC8247771.
[18]Ibid.
[19] Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020 Jun 30;25(2):113-123. doi: 10.3746/pnf.2020.25.2.113. PMID: 32676461; PMCID: PMC7333005.
[20] Georgiou K, Belev NA, Koutouratsas T, Katifelis H, Gazouli M. Gut microbiome: Linking together obesity, bariatric surgery and associated clinical outcomes under a single focus. World J Gastrointest Pathophysiol. 2022;13(3):59-72. doi:10.4291/wjgp.v13.i3.59; Hamamah S, Hajnal A, Covasa M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients. 2024;16(7):1071. Published 2024 Apr 5. doi:10.3390/nu16071071
[21] Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019 Jan 10;7(1):14. doi: 10.3390/microorganisms7010014. PMID: 30634578; PMCID: PMC6351938
[22] Mousa WK, Chehadeh F, Husband S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front Microbiol. 2022 Feb 3;13:825338. doi: 10.3389/fmicb.2022.825338. PMID: 35185849; PMCID: PMC8851206; Coussa A, Hasan HA, Barber TM. Impact of contraception and IVF hormones on metabolic, endocrine, and inflammatory status. J Assist Reprod Genet. 2020 Jun;37(6):1267-1272. doi: 10.1007/s10815-020-01756-z. Epub 2020 Mar 25. PMID: 32215823; PMCID: PMC7311610.
[23] Mousa WK, Chehadeh F, Husband S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front Microbiol. 2022 Feb 3;13:825338. doi: 10.3389/fmicb.2022.825338. PMID: 35185849; PMCID: PMC8851206; Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine. 2021 Apr;66:103293. doi: 10.1016/j.ebiom.2021.103293. Epub 2021 Apr 1. PMID: 33813134; PMCID: PMC8047503
[24] Martinez JE, Kahana DD, Ghuman S, et al. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front Endocrinol (Lausanne). 2021;12:667066. Published 2021 Jun 8. doi:10.3389/fendo.2021.667066
[25] Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res. 2011;2011:875125. doi:10.4061/2011/875125
[26] Kaliannan, K., Robertson, R.C., Murphy, K. et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;205. https://doi.org/10.1186/s40168-018-0587-0
[27] Park DY, Park JY, Lee D, Hwang I, Kim HS. Leaky Gum: The Revisited Origin of Systemic Diseases. Cells. 2022;11(7):1079. Published 2022 Mar 23. doi:10.3390/cells11071079
[28] Mori K, Yoshida K. Viral infection in induction of Hashimoto’s thyroiditis: a key player or just a bystander?. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):418-424. doi:10.1097/MED.0b013e32833cf518; Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993;14(1):107-120. doi:10.1210/edrv-14-1-107
[29] Mousa WK, Chehadeh F, Husband S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front Microbiol. 2022 Feb 3;13:825338. doi: 10.3389/fmicb.2022.825338. PMID: 35185849; PMCID: PMC8851206.
[30] Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020 Jan 31;11:25. doi: 10.3389/fendo.2020.00025. PMID: 32082260; PMCID: PMC7005631.
[31] Wang S, et al. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J Funct Foods. 2020; 66(1):103838 DOI:10.1016/j.jff.2020.103838; Feng W, Ao H, Peng C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front Pharmacol. 2018;9:1354. Published 2018 Nov 23. doi:10.3389/fphar.2018.01354; Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019;20(5):1214. Published 2019 Mar 11. doi:10.3390/ijms20051214; Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases [published correction appears in Front Immunol. 2019 Jun 28;10:1486]. Front Immunol. 2019;10:277. Published 2019 Mar 11. doi:10.3389/fimmu.2019.00277; Gagliardi A, Totino V, Cacciotti F, et al. Rebuilding the Gut Microbiota Ecosystem. Int J Environ Res Public Health. 2018;15(8):1679. Published 2018 Aug 7. doi:10.3390/ijerph15081679
[32] LaBouyer, M., et al. Higher total faecal short-chain fatty acid concentrations correlate with increasing proportions of butyrate and decreasing proportions of branched-chain fatty acids across multiple human studies. Gut Microbiome. 3, E2. doi:10.1017/gmb.2022.1; Liu S, An Y, Cao B, Sun R, Ke J, Zhao D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int J Endocrinol. 2020;2020:5036959. Published 2020 Nov 10. doi:10.1155/2020/5036959; Tottey W, Feria-Gervasio D, Gaci N, et al. Colonic Transit Time Is a Driven Force of the Gut Microbiota Composition and Metabolism: In Vitro Evidence. J Neurogastroenterol Motil. 2017;23(1):124-134. doi:10.5056/jnm16042
[33] Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases [published correction appears in Front Immunol. 2019 Jun 28;10:1486]. Front Immunol. 2019;10:277. Published 2019 Mar 11. doi:10.3389/fimmu.2019.00277; Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients. 2020 Apr 14;12(4):1082. doi: 10.3390/nu12041082. PMID: 32295104; PMCID: PMC7230435; Su X, Zhao Y, Li Y, Ma S, Wang Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin Sci (Lond). 2020 Jun 26;134(12):1521-1535. doi: 10.1042/CS20200475. PMID: 32519746; Bargiel P, Szczuko M, Stachowska L, et al. Microbiome Metabolites and Thyroid Dysfunction. J Clin Med. 2021;10(16):3609. Published 2021 Aug 16. doi:10.3390/jcm10163609; Li A, Li T, Gao X, Yan H, Chen J, Huang M, Wang L, Yin D, Li H, Ma R, Zeng Q, Ding S. Gut Microbiome Alterations in Patients With Thyroid Nodules. Front Cell Infect Microbiol. 2021 Mar 12;11:643968. doi: 10.3389/fcimb.2021.643968. PMID: 33791245; PMCID: PMC8005713.
[34] Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients. 2020 Jun 12;12(6):1769. doi: 10.3390/nu12061769. PMID: 32545596; PMCID: PMC7353203; Siddiqui MT, Cresci GAM. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021 Nov 18;14:6025-6041. doi: 10.2147/JIR.S300989. PMID: 34819742; PMCID: PMC8608412; Martin-Nuñez GM, Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Gut Microbiota: The Missing Link Between Helicobacter pylori Infection and Metabolic Disorders? Front Endocrinol (Lausanne). 2021 Jun 17;12:639856. doi: 10.3389/fendo.2021.639856. PMID: 34220702; PMCID: PMC8247771.
[35] Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, Murthy SN. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 2000;46(4):493-499. doi:10.1136/gut.46.4.493
[36] Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. Published 2020 Jan 31. doi:10.3389/fendo.2020.00025; Yagi A, Al-Madboly L, Kabbash A, El-Aasr M. Dietary Aloe vera gel and Microbiota Interactions: Influence of Butyrate and Insulin Sensitivity. Journal of Gastroenterology and Hepatology Research. 2017; 6(4): 2376-2383
[37] Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function?. Nutrients. 2020;12(6):1769. Published 2020 Jun 12. doi:10.3390/nu12061769
[38] Siddiqui MT, Cresci GAM. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021;14:6025-6041. Published 2021 Nov 18. doi:10.2147/JIR.S300989
[39] Bargiel P, Szczuko M, Stachowska L, et al. Microbiome Metabolites and Thyroid Dysfunction. J Clin Med. 2021;10(16):3609. Published 2021 Aug 16. doi:10.3390/jcm10163609; Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019;20(5):1214. Published 2019 Mar 11. doi:10.3390/ijms20051214
[40] Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice [published correction appears in Sci Transl Med. 2014 Dec 10;6(266):266er7. Guan, Ng Lai [corrected to Ng, Lai Guan]]. Sci Transl Med. 2014;6(263):263ra158. doi:10.1126/scitranslmed.3009759
[41] Zhang J, Song L, Wang Y, et al. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34(8):1368-1376. doi:10.1111/jgh.14536
[42] Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr. 2015;145(1):143-149. doi:10.3945/jn.114.198457
[43] Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509-1517. doi:10.2337/db08-1637
[44] Liu H, Wang J, He T, et al. Butyrate: A Double-Edged Sword for Health?. Adv Nutr. 2018;9(1):21-29. doi:10.1093/advances/nmx009
[45] Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases [published correction appears in Front Immunol. 2019 Jun 28;10:1486]. Front Immunol. 2019;10:277. Published 2019 Mar 11. doi:10.3389/fimmu.2019.00277; Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019;20(5):1214. Published 2019 Mar 11. doi:10.3390/ijms20051214
[46] Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1-24. doi:10.1007/s00394-017-1445-8
[47] Hsu SP, Chen HH, Wang TY, et al. Association of Hemorrhoids With Hashimoto’s Thyroiditis and Associated Comorbidities: A Nationwide Population-Based Cohort Study. Front Endocrinol (Lausanne). 2020;11:577767. Published 2020 Oct 8. doi:10.3389/fendo.2020.577767
[48] Lipopolysaccharide-induced inflammation. ScienceDirect . Accessed July 1, 2024. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/lipopolysaccharide-induced-inflammation.
[49] Mousa WK, Chehadeh F, Husband S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front Microbiol. 2022 Feb 3;13:825338. doi: 10.3389/fmicb.2022.825338. PMID: 35185849; PMCID: PMC8851206; Wassenaar TM, Zimmermann K. Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We be Worried?. Eur J Microbiol Immunol (Bp). 2018;8(3):63-69. Published 2018 Aug 21. doi:10.1556/1886.2018.00017
[50] Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021 Feb;78(4):1233-1261. doi: 10.1007/s00018-020-03656-y. Epub 2020 Oct 15. PMID: 33057840; PMCID: PMC7904555; Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145-151. doi:10.1016/j.cyto.2008.01.006
[51] Iaglova NV, Berezov TT. [Regulation of thyroid and pituitary functions by lipopolysaccharide]. Biomed Khim. 2010 Mar-Apr;56(2):179-86. Russian. PMID: 21341506.
[52] De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74-85. doi:10.1111/cei.13158
[53] Och A, Och M, Nowak R, Podgórska D, Podgórski R. Berberine, a Herbal Metabolite in the Metabolic Syndrome: The Risk Factors, Course, and Consequences of the Disease. Molecules. 2022;27(4):1351. Published 2022 Feb 17. doi:10.3390/molecules27041351; Li Y, Zhou J, Qiu J, et al. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 2020;261:118460. doi:10.1016/j.lfs.2020.118460
[54] Gong Y, Lu Q, Liu Y, et al. Dietary berberine alleviates high carbohydrate diet-induced intestinal damages and improves lipid metabolism in largemouth bass (Micropterus salmoides). Front Nutr. 2022;9:1010859. Published 2022 Sep 23. doi:10.3389/fnut.2022.1010859
[55] Elizondo-Luévano JH, Castro-Ríos R, López-Abán J, et al. Berberine: A nematocidal alkaloid from Argemone mexicana against Strongyloides venezuelensis. Exp Parasitol. 2021;220:108043. doi:10.1016/j.exppara.2020.108043
[56] Xie Y, Liu X, Zhou P. In vitro Antifungal Effects of Berberine Against Candida spp. In Planktonic and Biofilm Conditions. Drug Des Devel Ther. 2020;14:87-101. Published 2020 Jan 9. doi:10.2147/DDDT.S230857
[57] Zhang D, Ke L, Ni Z, et al. Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase IV trial. Medicine (Baltimore). 2017;96(32):e7697. doi:10.1097/MD.0000000000007697; Zhang L, Wu X, Yang R, et al. Effects of Berberine on the Gastrointestinal Microbiota. Front Cell Infect Microbiol. 2021;10:588517. Published 2021 Feb 19. doi:10.3389/fcimb.2020.588517
[58] Zhang L, Wu X, Yang R, et al. Effects of Berberine on the Gastrointestinal Microbiota. Front Cell Infect Microbiol. 2021;10:588517. Published 2021 Feb 19. doi:10.3389/fcimb.2020.588517
[59] Topping DL, Fukushima M, Bird AR. Resistant starch as a prebiotic and synbiotic: state of the art. Proc Nutr Soc. 2003;62(1):171-176. doi:10.1079/pns2002224
[60] Wang, X., Brown, I.L., Evans, A.J. and Conway, P.L. (1999), The protective effects of high amylose maize (amylomaize) starch granules on the survival of Bifidobacterium spp. in the mouse intestinal tract. Journal of Applied Microbiology. 87: 631-639. https://doi.org/10.1046/j.1365-2672.1999.00836.x
[61] Bodinham CL, Frost GS, Robertson MD. Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr. 2010;103(6):917-922. doi:10.1017/S0007114509992534
[62] Nugent, AP. Health properties of resistant starch. Nutrition Bulletin. 2005;30:27-54. https://doi.org/10.1111/j.1467-3010.2005.00481.x
[63] Kaur AP, Bhardwaj S, Dhanjal DS, Nepovimova E, Cruz-Martins N, Kuča K, Chopra C, Singh R, Kumar H, Șen F, Kumar V, Verma R, Kumar D. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules. 2021 Mar 16;11(3):440. doi: 10.3390/biom11030440. PMID: 33809763; PMCID: PMC8002343.
[64] Takahashi D, Hoshina N, Kabumoto Y, et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine. 2020;58:102913. doi:10.1016/j.ebiom.2020.102913
[65] Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases [published correction appears in Front Immunol. 2019 Jun 28;10:1486]. Front Immunol. 2019;10:277. Published 2019 Mar 11. doi:10.3389/fimmu.2019.00277
[66] Li A, Li T, Gao X, et al. Gut Microbiome Alterations in Patients With Thyroid Nodules. Front Cell Infect Microbiol. 2021;11:643968. Published 2021 Mar 12. doi:10.3389/fcimb.2021.643968
[67] Martinez JE, Kahana DD, Ghuman S, et al. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front Endocrinol (Lausanne). 2021;12:667066. Published 2021 Jun 8. doi:10.3389/fendo.2021.667066
[68] Food allergies can be reversed in mice by targeting the microbiome. American Chemical Society. August 21, 2022. Accessed July 25, 2024. https://www.acs.org/pressroom/newsreleases/2022/august/ food-allergies-can-be-reversed-in-mice-by-targeting-the-microbiome.html.

 Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
Hi, thank you for the article. How long should I také berberin for suspected gut dysbiosis , also how long is good to take butyrate? Thank you .
Lenka, I recommend discussing this with your practitioner to help you determine what will work best for you.