One night, I had a lucid dream that brought me to the past. For those of you who have never had a lucid dream, in this type of dream, you know that you are dreaming and, thus, you know that you will be safe… no matter what happens, or what you do. These dreams can be very liberating!
In this dream, I was taken back to a doctor’s visit back in 2006, when I first graduated from pharmacy school, and when I first began to have overt thyroid symptoms. In real life, I went in because I didn’t feel like myself, but my physical symptoms were ignored during this appointment. Instead of focusing on my neck pain, sore throat, palpitations, and fatigue, the doctor started narrowing in on my anxiety and obsessive-compulsive tendencies.
I was nervous and fidgeting… and somehow, we got on the topic of my habits.
I remember explaining that I triple checked prescriptions before I filled them (this was, after all, my job and requirement as a dispensing pharmacist) — but somehow, describing my daily job duties made me appear crazy to him. He kept digging further, trying to imply that everything physical that I was reporting was in my head.
I remember he asked me if I frequently washed my hands. I said, “No, but I would if I was preparing intravenous (IV) drugs!” (frequent handwashing is also a requirement for pharmacists that make IVs). I was anxious; I almost felt like he was quizzing me — and would report me if I didn’t wash my hands enough. I was a new grad and was used to being quizzed by older authority figures… and so of course, I looked even more crazy to him.
I knew that I was overly stressed due to being new to my profession, and I was a Type A personality and a perfectionist… but I didn’t feel like any of that was related to why I felt unwell with the many physical symptoms I had pointed to (and that were ignored) during my exam! I felt there was something going on in my body that was seriously affecting my health.
But the doctor’s recommended treatment? Antidepressants!
In my younger days when I was a rebel without a cause, I would have told him to shove it and that, as a trained pharmacist with a doctorate degree, I knew better. Instead, I was polite and sheepishly took home a prescription for antidepressants that I never filled.
You see, somewhere in my mid-20s, I began to censor myself. My teens and early twenties were all about seeking pleasure, being myself, and speaking my mind… but then it all changed.
I don’t know if it was my professional training, my friends, peers or partners, or simply growing older, but as time went on, I became pathologically polite. And so, even though my needs weren’t met, and even though I knew that my symptoms weren’t just in my head, I kept quiet. I didn’t push, and didn’t ask further questions.
Three years and many lingering physical symptoms later, I was found to have thyroid antibodies that were in the 2000+ range, and a thyroid gland so damaged that I needed to start thyroid medications.
Then, later down the road, I learned that anxiety, along with obsessive-compulsive symptoms and/or disorder (OCD) can often be early signs of Hashimoto’s. (In fact, the most common anxiety disorder reported in people with thyroid antibodies is OCD!)
Since then, I’ve had many new clients come to me with anxiety and mood-related symptoms who had been told that their TSH level was normal. They were also told that, instead of worrying about their thyroid, they should focus on their “psychiatric condition” or their “hypochondria”! Many had also been prescribed antidepressants.
When I had them tested with a full thyroid panel, most had thyroid antibodies.
Based on these clinical observations, it isn’t surprising to me that the research has found that people with thyroid antibodies will often have obsessive-compulsive disorder.
The good news is that there are many interventions (like treating Streptococcal-based gut infections, removing reactive foods, and addressing nutrient deficiencies affecting serotonin production) that can result in improvements to OCD, while reducing thyroid antibodies as well! (Coincidence? I think not!)
This article focuses on the strong link between Hashimoto’s (elevated thyroid antibodies) and having OCD or developing OCD tendencies. Interestingly, Strep infections have also been linked to both OCD and Hashimoto’s, and I’ll talk about that connection as well.
In today’s article, you will learn about:
- The many shades of obsessive-compulsive disorder
- What causes OCD
- The link between OCD and Hashimoto’s
- The conventional approach to OCD
- My root cause recommendations for OCD
The Many Shades of Obsessive-Compulsive Disorder
Obsessions are uncontrollable, recurring and irrational thoughts, urges, or images that lead to a strong emotional reaction such as anxiety, disgust, or distress. A common obsession is about the need for extreme cleanliness, or fear of contamination. Someone with an irrational need for cleanliness may become anxious when they perceive their house isn’t completely germ-free, or that everything isn’t in its precise place. This can lead to some of the most common compulsive behaviors or “rituals,” such as repetitive cleaning or reorganizing and ordering things. [1]
People who have obsessive-compulsive disorder typically have both obsessions and related compulsions, but sometimes they may only have one or the other. Symptoms can ease or worsen over time, and stress may exacerbate them.
Like many psychiatric disorders, there is a continuum of symptoms and behavior relating to OCD. On the one end, you have people who don’t fulfill all of the symptom requirements for a psychiatric diagnosis of OCD. This level of obsessive-compulsive behavior is sometimes referred to as subthreshold obsessive-compulsive disorder (OCD) behavior, or simply someone having obsessive-compulsive symptoms (OCS). [2]
We all know at least one person who has OCD tendencies such as perfectionism, safety-related rituals (checking locks prior to bedtime), or the need to adhere to strict routines. But most people like this are able to control going overboard on their emotional reactions or repetitive behaviors, and may only spend minutes on their thoughts (obsessions) and/or behaviors (compulsions) each day.
While subthreshold OCD might not be disabling, it may limit people in terms of their happiness and anxiety levels. It may also signal some underlying biological vulnerability (perhaps the root cause for future neurological distress or inflammatory states).
On the other end of the obsessive-compulsive continuum, you have the more serious, life-affecting condition of OCD that meets psychiatric diagnosis criteria.
Here, someone can suffer from agonizing obsessions that result in repetitive behaviors aimed at reducing the anxiety or distress associated with the thought or perceived dreaded situation. The compulsions do not necessarily have to be logical or even connected with the thoughts. The compulsions are likely to be excessive, ritualistic, and absolutely necessary for the person in order for them to neutralize their distress.
This level of OCD can result in a significant decline in a person’s ability to function, impede their social growth, and impact on their relationships, job opportunities, financial status, and overall quality of life. These people will likely spend at least one hour a day on their thoughts and/or behaviors.
Common examples are people who worry about cleanliness or disease and wash their hands until the point of rawness. Another example would be someone who can’t leave the house without checking every door and lock three times — or frankly, someone who can no longer leave the house due to some irrational fear (and who spends time, then, performing a ritual that may not have anything to do with that fear, like knocking three times on a particular door in their house). People suffering from this level of OCD may be fully aware that the behavior they are performing is abnormal and irrational, but they are unable to stop.
Some examples of extreme obsessions include fears of harm (of oneself or of harming others in an aggressive or horrific way), fears of contamination, sexual fears, religious fears, doubting oneself to the extreme, and the need to make things perfect, orderly, and symmetrical. [3]
Compulsions seen related to these obsessions can include checking/re-checking, repetitive behaviors related to cleaning, reassurance-seeking behaviors, rearranging and ordering things, hoarding, ritualistic behaviors having nothing to do with the fear, mental rituals (reciting words or numbers in one’s head), etc. There is often an inability to cope with uncertainty and a heightened sense of responsibility for what may happen to others. [4]
People with OCD are more likely to have a tic disorder (such as repetitive eye movements, shoulder or head jerking, facial grimacing, or repetitive vocal tics) than the general population. [5]
Diagnosing Obsessive-Compulsive Disorder
Most doctors will perform a physical exam to ensure a patient’s symptoms aren’t due to other reasons. This should include blood testing with a full thyroid panel.
There are psychological screening tools that evaluate a person’s feelings, fears, obsessions, and compulsive behaviors. Likely your doctor will use the diagnosis criteria for OCD found in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published by the American Psychiatric Association. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) is a screening tool that is typically used by clinicians, and self-report versions are available online.
A psychiatric diagnosis focuses on the degree of symptoms, whether an individual can control their thoughts and behaviors, how much time they spend on their thoughts and behaviors each day, how they view their own thoughts and behaviors, and whether they experience significant problems in their life due to their thoughts and behaviors.
The graphic below summarizes the diagnosis criteria for OCD, and shows that someone with subthreshold OCD will not have all of the requirements for a full OCD diagnosis. [6] Again, that doesn’t mean their health and lives aren’t being negatively impacted such that they’d benefit from treatment.
What Causes OCD?
Obsessive-compulsive disorder usually begins in late childhood/early adolescence or in early adulthood (typically by age 21). [7]
There is still a great deal of uncertainty relating to the exact causes of obsessive-compulsive disorder, but researchers agree there are multiple factors, including:
- Genetic predisposition and epigenetic influences
- Inflammation and immune system imbalances
- Neurotransmitter dysfunction
- Gut microbiome alterations
- Oxidative stress
Keep Hashimoto’s in mind as you think about these potential causes, since both conditions share many underlying commonalities. I’ll talk more specifically about Hashimoto’s and OCD in the next section.
Genetic Predisposition and Epigenetic Influences
Research has found that OCD involves biological disconnects in the communication between the front part of the brain and deeper structures of the brain (referred to as the cortico-striato-thalamo-cortical circuitry, or CSTC). [8]
There is also suspected dysregulation of neurotransmitters along this pathway, including serotonin, dopamine, and glutamine, as well as downstream effects of neurotransmitter signaling dysfunction on a number of growth and neurotrophic factors. [9] These structural and neurotransmitter predispositions are likely heritable. [10]
Having a first-degree relative with OCD increases the risk of developing the condition. Twin and family studies have found that the heritability appears to be as high as 45 to 65 percent in children, and 27 to 45 percent in adults. [11]
A separate study also concluded that subthreshold OCD (or having obsessive-compulsive symptoms, but not the full diagnosis) was also heritable, with a range from 30 to 77 percent. [12]
But what is being inherited?
There isn’t an “OCD” gene (just like there isn’t a single “Hashimoto’s gene”). While studies show the condition does run in families, there are likely a multitude of genes that could affect whether someone will exhibit OCD symptoms, as well as how their particular obsessive-compulsive symptoms will manifest.
Gene variations contributing to OCD may include those relating to the physical brain circuitry (CSTC), neurotransmitter synthesis and function, the body’s immune system, and endocrine system. Some gene variations have been identified, one example being a mutation in N-methyl-D-aspartate (NMDA) which affects receptors involved in central nervous system processing. This genetic mutation has been linked to an increase in the OCD-specific behavior of compulsive cleaning (related to the fear of contamination). [13]
Another example is a connection between OCD and dysregulated insulin signaling (likely due to gene variations that regulate central nervous system synaptic connections and function). One study found increased obsessive-compulsive symptoms in men with type 1 diabetes. Another found that OCD patients had a higher risk of developing type 2 diabetes. Obsessive-compulsive symptoms were also found to be positively correlated with blood levels of HbA1c, a commonly used diagnostic measure of type 2 diabetes. In one study, the severity of obsessive-compulsive symptoms decreased with better glycemic control. [14]
In identifying these connections, we are learning where lifestyle interventions such as addressing glucose control and insulin sensitivity issues may help ease OCD symptoms.
Neurological Disorders
Roughly 90 percent of those with OCD have coexisting psychiatric diagnoses, and the most commonly found ones are other anxiety disorders. [15] Other neurological disorders linked to OCD (involving the same brain circuitry) include Parkinson’s disease, Tourette syndrome, epilepsy, major depressive disorder, anorexia nervosa, and traumatic brain injury. [16]
Epigenetics
Even when gene variations are found that may predispose vulnerability to OCD, how those may be expressed in a given individual can be modified by epigenetics.
Epigenetics is where genes can be modified by environmental factors (basically turning them on or off), including infections, toxin exposures, stress, and childhood trauma as a few examples. Familial risk, then, represents more than just an underlying genetic vulnerability, but possibly an increased risk due to familial clustering of unfavorable family circumstances (poor diet, toxins, etc.) in early life.
We see this in Hashimoto’s as well, where the genetic heritability is around 25 percent (and also involves a multitude of different gene variations), but epigenetics and environmental factors likely increase that risk to 75 percent. The great news here is that much of this 75 percent consists of factors that we have control over changing!
Stress
Stress in particular has been investigated relating to OCD onset. Certainly, trauma at a young age has been implicated, but everyday types of stress have increasingly come onto the radar. There have been fascinating findings in recent research, including a positive association found between individuals having a stress-related event preceding the onset of OCD. In one study, the types of stressful triggering events most reported were social/family relationship stressors (15.4 percent), health problems (15.4 percent), education (14.1 percent), and work difficulties (12.1 percent). [17]
Inflammation and Immune System Imbalances
Ongoing inflammation (from increased levels of cytokines and inflammatory agents), co-occurring autoimmune and neuro-inflammatory disorders (evidence of antibodies), and triggering events (like infections and stress) have been observed in OCD. [18]
Scientists first looked at the immune system connection in a condition known as PANDAS (pediatric autoimmune neuropsychiatric disorders), where children developed OCD symptoms after having Streptococcus infections (the bacteria that results in the common throat infection known as “strep throat”). [19]
Research has found that Streptococcus is involved in a few different diseases, including rheumatic fever, scarlet fever, Sydenham chorea, PANDAS, and OCD. [20] Some more recent data suggests that a Streptococcal infection may play a role in patients with OCD, even in the absence of the other diseases mentioned.
A cross-sectional study found that in patients with a history of rheumatic fever, the rate of clinical OCD and subclinical OCD was 10 and three percent, respectively, which is much higher than the one to three percent rate of OCD reported in the general population. [21]
Another study found that individuals with a positive Streptococcal test had an increased risk for any mental disorder, especially OCD and tic disorders. [22]
Case reports have shown that adults can develop obsessions and compulsions after severe sore throat and fever, which suggests that there may be a PANDAS-like effect in adults as well as children. [23]
Yet another study revealed that patients younger than 18 years old who had moderate or high frequencies of Streptococcus-related clinic visits, had much higher risks of developing a neuropsychiatric disorder later in life. [24] They especially had a higher risk of developing tic disorders and OCD.
Researchers believe that the infection, through molecular mimicry, results in autoimmune antibodies against the basal ganglia (subcortical structures found deep within the brain), leading to brain neuro-inflammation, and triggering obsessive thoughts and compulsive behaviors. Molecular mimicry is also a leading theory for autoimmune diseases such as Hashimoto’s. Basically, the body’s immune system begins to attack itself (the thyroid is targeted in the case of Hashimoto’s) when we are infected with an invading pathogen (like an infection) that looks similar to the thyroid in terms of its protein sequences (kind of a case of mistaken identity).
Lots of children get Strep infections, but not all develop psychiatric symptoms, so it is important to always remember there is a genetic vulnerability that predisposes people to develop various conditions of immune dysregulation. This is true in Hashimoto’s as well.
After PANDAS was initially identified, other diseases affecting the immune system and inflammatory response were also linked to OCD behavior. The brain circuitry mentioned earlier, in particular, has shown an inflammatory response associated with OCD, with the degree of inflammation found to be associated with the ability of an individual to resist their compulsions. [25]
There has been a strong link identified between OCD and autoimmune disorders. Mothers of individuals with OCD have been found to have elevated rates of autoimmune diseases, including Hashimoto’s. Studies have shown that 43 percent of people having OCD are likely to have an autoimmune disease with some of the strongest associations being celiac disease (76 percent), Hashimoto’s (59 percent), and Type 1 diabetes mellitus (56 percent). [26]
I’ll talk more about the connection between OCD, thyroid antibodies, and Hashimoto’s in a moment.
Neurotransmitter Dysfunction
The specific CSTC circuitry involved in OCD is responsible for neurotransmitter synthesis, including serotonin, dopamine, and glutamate. Two neurotransmitters in particular are involved in the etiology of OCD: serotonin and glutamate.
Serotonin
Serotonin, known as the “happy” hormone, is responsible for the positive sensations associated with relaxation, satisfaction, and satiety. It also helps regulate appetite and helps us sleep (via its conversion to melatonin). It is thought to be dysregulated in OCD. [27]
Studies have shown that reductions in serotonin make subjects more sensitive to stress. [28] The interaction between the HPA axis and serotonin synthesis may play a role in increasing some people’s genetic vulnerability to stress-related onset of OCD. [29]
While the research points to serotonin dysregulation as a likely cause of OCD, most of the findings are suggestive and not directly linked. The reason for this is that there is currently no simple way to measure levels or the activity of brain serotonin around synapses in humans. One compelling argument in favor of serotonin as a cause, however, continues to be the therapeutic use of selective serotonin reuptake inhibitors (SSRIs) as the primary medical treatment option to address OCD (while other antidepressants have typically not proven to be effective). [30]
While SSRIs do not work for everyone, they are still the primary treatment option, and they work by blocking the action of the serotonin reuptake transporter, making more serotonin available.
Another supportive clue that serotonin is key to OCD is found in studies related to the many nutrients and amino acids necessary for proper serotonin synthesis (zinc, magnesium, B6, and tryptophan to name a few). Deficiencies in these nutrients have been linked to mood issues, anxiety, and even OCD itself. [31] Is it coincidence that many of these nutrients are often deficient in people with Hashimoto’s? I think not!
Another interesting link to serotonin dysregulation involvement in OCD can be found in studies on estrogen. Estrogen interacts with the serotonergic system and appears to enhance serotonin signaling and be neuroprotective — so we would expect to see improvements in OCD symptoms when estrogen levels are higher, and detriments when estrogen levels are low. [32]
Research has found that there is an exacerbation of preexisting OCD symptoms and increased incidences of OCD in females during reproductive events that are related to low levels of estrogen — for example, early in pregnancy, during postpartum, and about four days prior to menstruation. One 2013 analysis found that pregnant women had a 45 percent increased risk, and postpartum women had a 138 percent increased risk of developing OCD! [33]
It’s worth noting that 95 percent of serotonin is actually synthesized in the gut… and most of the body’s immune system is also in the gut. We’ll see in the next section how the gut microbiota are thought to impact neurotransmitter activity, and how specific gut bacteria such as Strep have been identified as common in Hashimoto’s. Gut imbalances, leaky gut, and infections are known triggers in Hashimoto’s. (Another coincidence? Hmmmmm…)
Glutamate
Scientists are also looking at the role of glutamate in relation to OCD.
Glutamate is a major excitatory neurotransmitter involved in a variety of central nervous system functions. Along with serotonin, it is one of the main neurotransmitters within the CSTC brain circuitry involved with OCD. High levels of glutamate have been implicated in OCD as well as a number of other neuropsychiatric disorders, including schizophrenia, Alzheimer’s disease, Parkinson’s disease, and epilepsy. [34]
In studies (cerebrospinal fluid studies have provided the most direct evidence) looking at the association between glutamate and OCD, researchers have found evidence of glutamatergic dysfunction, with excessive amounts found in a subset of patients. [35] Genetic studies have also found glutamatergic dysfunction in some OCD patients, and correlated specific glutamatergic genes with OCD. [36]
Glutamate is a precursor for gamma-aminobutyric acid (GABA, our calming hormone), and the two work together in building the CSTC brain circuitry; when these are out of balance, which can happen if there is an excess of glutamate, for instance, the result may be dysfunctional development of this OCD-related brain circuitry.
Glutamate has been found to play a role in fear extinction, which is impaired in OCD. [37] It also plays a role in immune modulation, and may be responsible for inflammatory processes which result in OCD in some cases. [38]
Further evidence of its role in OCD is seen in the effective use of glutamate-modulating medications in a subset of people, focused on reducing the release of glutamate. [39]
But do you know what helps to break down glutamate, make neurotransmitters, and has also been studied in OCD? Vitamin B6! 🙂
As you can see in the graphic below, the breakdown of glutamate and its synthesis into GABA is dependent on vitamin B6, so deficiencies in this nutrient may result in excess levels of glutamate. [40] It’s also needed for the synthesis of other neurotransmitters like dopamine and serotonin.
Vitamin B6 has been studied as an adjunct therapy to medications for managing symptoms of OCD. One study found co-administration of B6 with clomipramine or venlafaxine to be effective in enhancing the benefits of the drugs in OCD symptoms. [41]
P5P is the active form of vitamin B6, and the form I usually recommend for supplementation. In fact, I often recommend P5P for those with glutamate or oxalate issues. P5P can be taken at a dose of 50 to 200 mg/day. High-quality options of P5P include Rootcology P5P and one from Pure Encapsulations. (Please note, do not use more than 300 mg/day of B6 in the pyridoxine form, as it has been associated with neuropathy.)
Gut Microbiome Alterations
Given that most serotonin is produced in the gut, the gut microbiome has a significant influence on the serotonergic system as well as other neurotransmitters; therefore, it is key to the normal functioning of the gut-brain axis (the bi-directional system linking the brain and gastrointestinal tract), as well as the pathophysiology of neuropsychiatric conditions, including OCD and neurodevelopmental disorders.
There are a number of animal and laboratory studies where the gut microbiota have been manipulated, resulting in anxiety and OCD-like behavior. There are also studies showing that these OCD behaviors were reduced by pre-treatment with certain probiotics (such as Lactobacillus rhamnosus) and fluoxetine (an SSRI). [42]
I’ll talk a bit more about the link between the gut microbiome (including Strep overgrowth in the gut), Hashimoto’s, and OCD in the next section.
Oxidative Stress
In simple terms, oxidative stress is when we have reduced antioxidant defenses, and this results in an increased number of free radicals. High levels of free radicals can result in decreased synaptic performance (where neurotransmission, or communication between brain cells, occurs), and have been associated with both OCD and major depressive disorder. [43]
Research has found an increase in oxidative stress and decrease in antioxidants in people diagnosed with OCD. Hyperthyroidism and hypothyroidism have also been associated with greater levels of oxidative stress; thus, people with thyroid conditions are more at risk for antioxidant deficiencies. The good news is that we can address this! [44]
Another driver of oxidative stress to consider is homocysteine. Elevated homocysteine levels signify increased levels of inflammation, and have been linked to a number of health conditions, including heart disease, stroke, Alzheimer’s disease, and osteoporosis. [45]
Research has found that those with OCD have significantly higher levels of homocysteine than the general population, so this is an important factor to take into account when we’re looking at the picture of oxidative stress as it relates to OCD. [46]
Vitamin B12 was also found to be significantly lower in people with OCD, which may be connected to homocysteine levels via the MTHFR gene. The MTHFR gene variation is associated with a buildup of homocysteine due to impaired methylation, and B12 is needed to break down homocysteine. [47] Low levels of vitamin B12 are typically present with high homocysteine levels and the MTHFR gene variation.
To take it one step further, there may be a link between hypothyroidism and the MTHFR gene variation. While some studies have found the rates of this gene variation to be the same in the general population as it is in those with autoimmune thyroid disease, a 2020 study of 34 women with hypothyroidism actually found that the MTHFR gene variant is significantly associated with hypothyroidism. [48]
As mentioned, those with thyroid conditions are found to have lower levels of antioxidants and higher levels of oxidative stress, and the mechanisms mentioned above may shed light on what connects and drives both hypothyroidism and OCD. The next section explores this connection in-depth.
The Link Between Hashimoto’s and OCD
Hypothyroidism is associated with a number of diverse neuropsychological and psychiatric disorders with increased rates of obsessive-compulsive symptoms found in people with thyroid disease. OCD symptoms (both diagnosed OCD and threshold OCD) have been observed 1.4 times more in those with thyroid diseases (both non-autoimmune and autoimmune thyroiditis) than in the general population. [49]
Accumulating evidence points to dysfunction of the immune system as a potential factor in the development of mental disorders, including OCD.
A 2022 review found that almost 40 percent of OCD patients have an autoimmune disease. [50] Two studies in the review found that OCD appears to be more common in autoimmune thyroid diseases, like Hashimoto’s and Graves’ disease, than other thyroid disorders. There are also cases of OCD in papillary thyroid cancer, which is thought to be driven by immunological factors.
Another study in the review reported a higher rate of OCD in patients with thyroid diseases such as Graves’ disease, multinodular goiter, adenoma, and thyroid cancer, than in the general population.
Overall, OCD rates are higher in those with autoimmune thyroid disorders than in the general population.
I’ll overview the theories as to why this connection exists and appears to be so strong, particularly in the autoimmune hypothyroid condition Hashimoto’s. They include:
- Biochemical abnormalities
- Neuro-inflammation
- The autoimmune (thyroid antibodies) connection to OCD
- Triggers (and treatments!) in common
- Strep, OCD, and Hashimoto’s
- The gut microbiota-brain connection
Biochemical Abnormalities
Studies have found that euthyroid Hashimoto’s, meaning elevated thyroid antibodies with normal thyroid function, induces neuroinflammation and affects serotonin signaling. [51] This can result in more anxiety and depressive-like behaviors. Thyroid hormones themselves are also associated with mental health challenges. One study found that free T4 levels could serve as an independent biomarker related to anxiety and depression in people with autoimmune conditions. [52]
Neuro-inflammation
Both animal studies and human studies have found that Hashimoto’s induces neuroinflammation. Hashimoto’s increases inflammatory cytokines which may impact neurotransmitter synthesis, release, and uptake, affecting brain neural circuitry and resulting in emotional and mental impairments. [53]
It’s not just thyroid hormones that drive this process — it’s more likely that thyroid antibodies and the autoimmune process are impacting changes in the brain. In mice studies, Hashimoto’s has been shown to induce damage to parts of the brain that impact hippocampal-dependent learning and memory function. [54] The mice were in a euthyroid state, suggesting that the antibodies that are present in Hashimoto’s are what drive the damage in the brain. Other studies have shown that thyroid antibodies have the ability to bind with astrocytes (specific types of cells that are critical for nervous system function), and this process has the potential to alter neurological processes. [55]
Neuroimaging results of euthyroid Hashimoto’s patients (even those not exhibiting psychiatric symptoms) reveal cerebral perfusion impairments, particularly in a key region known for the control of emotional behaviors, the frontal cortex. [56]
So no wonder you may feel a bit crazy or overwhelmed at times… I know I did too before I became a Root Cause rebel and implemented my protocols, including a focus on reducing thyroid antibodies!
The Autoimmune (Thyroid Antibodies) Connection to OCD
Elevated antibodies in a wide variety of conditions are a key biomarker associated with OCD. [57] Studies have found that some 43 percent of people with OCD are likely to have an autoimmune disease, with 59 percent of that population having an increased risk for Hashimoto’s. [58]
I find it interesting that OCD is often the first symptom people may experience with Hashimoto’s. Trudy Scott, a wonderful nutritionist who specializes in anxiety and mood disorders, reports that up to 50 percent of her clients with anxiety have Hashimoto’s.
Thyroid antibodies in particular have been associated with increased frequencies of mood disorders. For example, in a 2004 study, an association was reported between the presence of an anxiety or mood disorder, and the presence of anti-TPO antibodies seen in Hashimoto’s. [59]
More specifically, a 2012 study found that the presence of TPO antibodies is associated with obsessive-compulsive symptoms, even after adjustment for age, gender, and thyroid function as assessed by TSH levels. [60]
Another study focusing on OCD found that patients with TPO antibodies had a greater number of inflammatory cytokines activated that impacted neurotransmitter metabolism. Cytokines were found to deplete tryptophan (an amino acid precursor of serotonin) as well as impact the regulation of dopamine and glutamate. Patients with thyroid antibodies had worse outcomes in terms of obsessive-compulsive symptoms. [61]
I do recall that when I was early in my Hashimoto’s journey, my own OCD tendencies would flare up at times when my thyroid antibodies were on the rise, and then those symptoms would reduce as my antibodies dropped!
Hashimoto’s and OCD: Common Triggers and Treatments
OCD and Hashimoto’s have many triggers in common, and these triggers have the potential to exacerbate both conditions. They include stress, high levels of estrogen, blood sugar, nutrient deficiencies (in particular those dealing with neurotransmitters), amino acid imbalances, and infections, to name a few.
The great news here is that many treatments may reduce both thyroid antibodies as well as OCD symptoms!
Strep, OCD, and Hashimoto’s
There is a growing body of evidence supporting the idea that Strep infections have the potential to trigger OCD. [62] One theory for this process is that Strep bacteria “hides” from the immune system by mimicking host cells. In some cases, this can cause antibodies to mistakenly attack a part of the brain that is involved in movement and behavior, which leads to the development of OCD, tics, and changes in handwriting and attention.
I talked earlier about the connection between OCD and Strep in PANDAS, so let me talk a bit about Strep, OCD, and Hashimoto’s. In my clinical practice with my Hashimoto’s clients, I’ve found a correlation between Strep overgrowth in the gut and OCD symptoms.
There is an entire field of research called immuno-neuropsychiatry, which focuses on the “gut-brain” axis. It looks at how chronic inflammation, autoimmunity, gastrointestinal health, and psychological stress can impact neurological health and psychiatric disorders. PANDAS, based on a Streptococcus pyogenes infection, immune system activation, and an OCD outcome, is one example of immune-neuropsychiatry in action. Hashimoto’s is another one.
I personally think that Strep is a trigger for Hashimoto’s, for four reasons:
- The degree to which I have seen Streptococcus bacteria overgrowth in my client population:
- In recent years, my team and I analyzed data collected from 298 people with Hashimoto’s who took the GI-MAP stool test (to identify gut pathogens). Fifty-eight percent of our samples had some level of Streptococcus (although only about 25 percent had abnormal levels based on conventional lab ranges). You can learn more about our findings in this article.
- While I haven’t analyzed all of my past clients’ intakes, I do recall having many clients denote Strep infections on their health history intake forms, just like other infections such as H. pylori, Epstein-Barr, and Yersinia enterocolitica (all known to trigger Hashimoto’s).
- There is research that shows that sleep apnea can be caused by Strep (the bacteria release toxins within the tonsils, causing them to become inflamed and swollen, triggering sleep apnea) — and sleep apnea is common in Hashimoto’s. [63] Myself and some of my fellow integrative practitioners view the tonsils as a protective barrier to the thyroid (they share similar blood supplies). So it’s not a far stretch to believe that Strep in the tonsils could travel to the thyroid.
- Genetic vulnerability of the immune system: While I haven’t found specific research linking Strep and Hashimoto’s, theoretically, like in PANDAS, if someone has genetic vulnerability to immune-related responses (such as people with autoimmune conditions), antistreptococcal antibodies could cross-react with the area of brain circuitry prone to OCD (just like in PANDAS). [64]
- The gut-brain connection and the high incidence of gut dysbiosis in Hashimoto’s: Gut microbiome imbalances, leaky gut, and other GI disorders (including IBS and IBD), as well as infections, have been shown to cause anxiety, cognitive dysfunction, and depression. [65] I’ll talk more about this next.
- Strep-targeted treatments help with OCD symptoms: I have seen treatments that target Strep, such as berberine, to be incredibly helpful for Hashimoto’s and reducing thyroid antibodies (hint: they’re also helpful for OCD). [66] Additionally, berberine is helpful for balancing blood sugar, which can help alleviate symptoms of both Hashimoto’s and OCD. [67]
The Gut Microbiota-Brain Connection
In Hashimoto’s, gut dysbiosis is very common due to:
- Root causes such as food sensitivities, toxins (and a liver backlog), infections, stress, and nutrient deficiencies, which can result in gut microbiome imbalances and leaky gut.
- The very common issue of having low stomach acid, which affects nutrient absorption, increases risk for food sensitivities, and again makes the gut more prone to infections.
Gut dysbiosis can cause an increase in short-chain fatty acids that may activate microglia cells (the immune cells of the central nervous system), leading to an increase in inflammatory cytokines that may alter brain connections and normal neuroendocrine regulation. This has been hypothesized to play a causative role in anxiety and mood disorders. [68]
When our gastrointestinal system is more prone to infections such as Strep, this may impact neurotransmitter synthesis, resulting in mental health symptoms. [69]
If you want to read more about gut dysbiosis, here are two Pubmed articles: Dysbiosis of the Gut Microbiota in Disease and The Gut Microbiome and the Brain. Please also refer to my recommendations in the linked article on the importance of gut health in Hashimoto’s.
The Conventional Approach to Addressing OCD
OCD is most commonly treated with the tricyclic antidepressant clomipramine or selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine, sertraline, paroxetine, fluvoxamine, escitalopram, citalopram, and venlafaxine.
There are a number of potential side effects, including headache, nausea, insomnia, nervousness or restlessness, drowsiness, sexual problems (reduced libido, erectile dysfunction, etc.), appetite loss, weight change, tardive dyskinesia, and dystonia.
Medication is usually paired with psychotherapy. Cognitive behavior therapy (CBT), a type of psychotherapy, attempts to break the associations between an individual’s sensations of distress and the thoughts that produce them (for instance, the thought of contamination), as well as break the association between distress and particular ritualistic behaviors (for instance, washing one’s hands over and over). A component of CBT that is sometimes used is exposure and response prevention (ERP), where an individual is gradually exposed to a feared obsession and learns to resist the urge to perform their compulsion ritual.
While a standard treatment, estimates suggest that around 30 to 60 percent of patients do not improve or show a partial response to this serotonergic-focused treatment. [70]
More recently, many glutamate-modulating drugs (memantine, lamotrigine, topiramate, riluzole, NAC, D-cycloserine, ketamine) have been reported to show positive results in the treatment of OCD in some people, and this therapeutic effect can be considered further evidence for the abnormal elevation of glutamate in patients with OCD. [71]
For most people, OCD is a chronic disorder, and long-term (even lifelong) therapy may be required. Relapse is common. There are more extreme treatments, including deep brain stimulation, transcranial magnetic stimulation, and plasma exchange.
Conventional medicine does not look at underlying nutrient deficiencies (again, there are many that affect neurotransmitter synthesis as well as gut health), nor lifestyle interventions that may address potential root causes for OCD.
The Root Cause Approach to Addressing OCD
While SSRI medications always have their place and may be necessary in some cases, it’s important to note that most of them do not help Hashimoto’s, and do not get to the root cause. Because having OCD or OCD tendencies is commonly reported by my clients (and something I have personally faced), I wanted to create a root cause approach to help.
Optimize Thyroid Hormone Medications
In a survey I did in 2015 with over 2000 people with Hashimoto’s, data showed that having a TSH level between 1 and 2 μIU/mL, and in some cases less than 1 μIU/mL, helped improve mood symptoms.
I have also found that, for many, T4-only medications sometimes simply do not work well, so I often recommend clients with anxiety, mood disorders, or OCD symptoms change their thyroid medication by adding a T3-containing medication. In my survey, 60 percent of respondents reported improved mood after switching to a T3-containing medication. You can learn more about T3 and T3/T4 combination medications here.
Check and Reduce Thyroid Antibodies
Antibodies are related to overall inflammatory levels and OCD symptoms, mood disorders, and a wide variety of autoimmune-related symptoms (including active thyroid damage). Know your levels and then work to reduce them!
I have many easy-to-implement protocols for reducing thyroid antibody levels that may help with OCD symptoms, too. Check out the linked article, and the remaining strategies listed here may also help with symptoms.
The following tests can help you check for antibodies:
Alternatively, you can opt for a full thyroid panel which includes tests for TSH, free T3, free T4, and two of the thyroid antibodies (TPO and TG).
Swap to an Anti-Inflammatory (Anti-OCD!) Diet
A Mediterranean-style diet contains nutrients supportive of brain function, including antioxidants like vitamins A, C, and E, B vitamins, vitamin D, omega-3 fatty acids, and many minerals important to neurotransmitter synthesis and function, including magnesium, zinc, iron, selenium, potassium and iodine. [72]
For Hashimoto’s, different diets can be helpful, but generally, a Paleo-style diet that focuses on an abundance of whole foods works well for many people. The Paleo diet eliminates gluten, dairy, and soy, all of which are common food sensitivities for those with Hashimoto’s. I also recommend a blood sugar-balancing diet for those with Hashimoto’s, as keeping blood sugar levels can support adrenal health and inflammation levels. You can support healthy blood sugar levels by focusing on protein and healthy fats at meals, while reducing your overall consumption of carbohydrates (especially processed carbohydrates).
These diet principles that are supportive of Hashimoto’s will also support the brain because they include the crucial nutrients listed above. To learn more about the best diet for Hashimoto’s, read my article here.
Remove Common Food Sensitivities Such as Gluten
In my experience, many people with Hashimoto’s have food sensitivities. Research suggests that a gluten-free diet improves OCD symptoms in children and adults. [73] In my survey, 60 percent of people reported improved mood by eliminating gluten, 59 percent had improvements by going grain-free, and 45 percent by giving up dairy.
Follow a Low-Glutamate Diet
Medications that modulate glutamate have shown effectiveness in treating OCD in certain populations of people. Glutamate can be found in foods in two forms: bound and free. It is having an excess of the free form that causes problems.
In one case study, restricting the intake of free glutamate resulted in the resolution of all the patient’s OCD symptoms which had been previously unresponsive to medication. [74] Foods containing high levels of free glutamate include: bone broth, soy-containing sauces like soy sauce, Asian fish sauce, and oyster sauce, aged cheeses like parmesan, and many types of flavor-enhancing food additives such as MSG, mushrooms, and tomato sauce. (As a related aside, although the amino acid glycine has been shown in one study to improve OCD symptoms, I don’t usually recommend it, as I’ve seen it convert to glutamate. There is also the potential for significant nausea as a side effect, which is why many people dropped out of the particular study.) [75]
Something else to consider when it comes to glutamate is that an excess conversion of glutamine to glutamate can occur when there is a deficiency of vitamin B6, which many people with Hashimoto’s tend to be deficient in. I always recommend the active form of vitamin B6, P5P.
Gain Improved Control Over Your Blood Sugar
There is research showing a connection between poor blood sugar control and OCD. (Blood sugar imbalances are also a very common inflammatory trigger for Hashimoto’s, resulting in a greater number of antibodies as well.) [76] In my survey, 61 percent of people reported an improved mood with a low glycemic index diet, while 65 percent experienced improvement on a sugar-free diet.
I often get asked about the keto diet, as it can be used therapeutically for a number of health conditions, including mental health issues like depression and psychosis. [77] Anecdotally, I have also seen the keto diet help with anxiety and OCD, likely because of its positive effects on blood sugar. While keto is controversial in the thyroid world, a dairy-free version is one of the diets that has personally helped me the most.
Magnesium, myo-inositol, milk thistle, and zinc are a few nutrients that have been shown to help stabilize blood sugar (and you’ll see below they are beneficial in addressing OCD as well!).
Improve Your Stress Response
We know stress is implicated in OCD and is a very common trigger for Hashimoto’s. There are many lifestyle interventions to help you better manage your stress response (as a busy mom, I realize we can’t often remove the stressors!). The linked article will provide you with many strategies for supporting stress.
I love sauna therapy — and in my survey, some 74 percent of people said that a sauna helped with their mood! (Please check out the linked article, however, for precautions.) I also love adrenal adaptogens, which are thought to normalize the HPA axis (and many are neuroprotective). One of my favorite adaptogens is ashwagandha, which has been shown to help normalize thyroid hormone levels and support the body’s stress response. Research has also suggested it may be neuroprotective. [78] In my 2015 survey, some 77 percent of people said their mood improved when they tried adaptogenic herbs such as ashwagandha.
Replenish Key Nutrients
Some key nutrients required for healthy neurotransmitter synthesis and function may also help to reduce thyroid antibodies.
Serotonin synthesis depends on the availability of the amino acid L-tryptophan and many essential cofactors, including vitamin B3, vitamin B6, folate (5-MTHF), and zinc. There are many nutrients important in modulating glutamate as well. Here are a few of the most common ones, which also happen to be common deficiencies in those with Hashimoto’s:
- Zinc helps modulate excess glutamate, is important to serotonin synthesis, and may help balance blood sugar. [79] Zinc has been found to improve outcomes for OCD treatment. [80] Consider zinc picolinate by Pure Encapsulations, because of its improved absorption profile compared to other forms. I usually recommend doses of no more than 30 mg per day, unless you’re working with a practitioner who advises higher doses.
- Magnesium blocks excess glutamate, and magnesium deficiency has been linked to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and symptoms such as anxiety. [81] It can also help balance blood sugar. [82] If you have Hashimoto’s and constipation, consider Magnesium Citrate Powder by Rootcology. If you tend towards diarrhea, consider Magnesium Glycinate by Pure Encapsulations. This type of magnesium has been shown to relieve magnesium deficiency on blood tests, but does not loosen stool. However, for some people, magnesium glycinate can worsen anxiety symptoms.
- B6 is highly associated with anxiety and OCD, supports serotonin and norepinephrine (your flight or fight response) synthesis, central nervous system functioning, and our circadian rhythm and sleep cycle (one interesting clue you are deficient in B6 is poor dream recall!).
Another very important function of B6 that relates to OCD specifically, is that it is necessary for the breakdown of glutamate. [83]
Vitamin B6 also helps reduce homocysteine levels. Elevated levels of homocysteine have been shown to be neurotoxic, as when it builds up, it inhibits methylation (which is the body’s detoxification process) and increases oxidative stress. Many people with hypothyroidism already have the biochemical abnormality of poor methylation, and individuals with the MTHFR gene variation also have poor methylation, resulting in elevated homocysteine levels and vitamin deficiencies required for neurotransmitter synthesis such as low folate. [84]
I often recommend the activated P5P version of vitamin B6, at a dose of 50-100 mg per day.
- Other B vitamins: Aside from B6, other B vitamins such as B3, B9, and B12 in particular are very involved in brain function. They are crucial for healthy levels of neurotransmitters, and influence homocysteine levels. [85] Treatment with B12, B6, B9 (folate), and B1 (thiamine) has been found to help reduce homocysteine levels and reduce OCD symptoms. A few other notes on the B vitamins:
- Vitamin B12: In my survey, 56 percent of people experienced improved mood when taking B12. Some researchers have speculated that because of vitamin B12’s role in neurotransmitter synthesis, OCD can sometimes be an early manifestation of a vitamin B12 deficiency. [86] To find out if you have low levels of B12, you can ask your health care provider for the B12 (or cobalamin) test. Alternatively, you can self-order the B12 test via Ulta Lab Tests. When you receive your test results, it’s important to note that optimal B12 levels should be between 700-900 pg/mL. As mentioned above, most labs will not flag B12 levels unless they are under 200 pg/mL. Please note that testing may not always be reliable, as the consumption of bread and cereals (or supplements) fortified with folic acid (synthetic folate) may mask a B12 deficiency on standard lab tests. My favorite B12 supplement is Pure Encapsulations Adenosyl/Hydroxy B12 or liquid Pure Encapsulations B12. Both can be administered sublingually for optimal absorption.
- Vitamin B3 (niacin): B3 increases tryptophan, a precursor in the production of serotonin.
- Vitamin B9 (folate): Helps ensure adequate levels of L-methylfolate (5-MTHF), the enzyme responsible for converting the amino acid tryptophan to 5-HTP, so it is critical for serotonin synthesis. Note that you want to supplement with folate versus folic acid. Folic acid requires an additional processing step and may result in folate deficiency, impacting serotonin. [87] Note that folate deficiency is common, and there are also many medications that increase the risk for its deficiency, including antacids, oral contraceptives, antibiotics, anticonvulsants, and Metformin. [88]
- Myo-Inositol: One of the nine forms of inositol, myo-inositol has been heavily studied for its benefits related to anxiety and OCD disorders, Hashimoto’s and thyroid disease, polycystic ovary syndrome (PCOS), and blood sugar, as well as its role as an antioxidant. This is a wonderful supplement to take in tackling any of those issues, and I often recommend it, particularly when someone with Hashimoto’s is experiencing OCD symptoms.
Myo-inositol appears to reverse the desensitization of serotonin receptors, improving serotonin availability. It has been studied, often in conjunction with selenium (more on selenium in the next section), as a beneficial treatment for reducing thyroid antibodies. There is also research relating specifically to OCD. In one study, the use of 18 grams of inositol alone significantly reduced OCD symptoms compared to placebo. It has proven to be helpful for SSRI-resistant patients, at least as a stand-alone therapy. [89]
According to integrative psychiatrist Dr. James Greenblatt, even though research studies suggest 18 grams of inositol per day, he starts all patients with OCD on approximately 3 grams of inositol per day. This minimizes GI side effects, which may include bloating and nausea. If needed, inositol dosages can be titrated up slowly, with most patients responding below 12 grams per day. [90] Rootcology makes a Myo-Inositol Powder that is easy to take (BONUS: get 10% off with code MYOINOSITOL10 now through January 17th at 11:59 pm PT). Please note, if you have kidney disease or are taking diuretic medications, please check with your practitioner before taking myo-inositol. Learn more about other precautions and information relating to myo-inositol at the linked article.
- Selenium: This is one of the most common deficiencies in Hashimoto’s. It is an important nutrient required for optimal T4 to T3 thyroid hormone conversion and is important for immune health. Low levels have been associated with anxiety symptoms and a reduced sense of well-being. Most importantly to thyroid health, selenium can also greatly reduce thyroid antibodies. One study showed that thyroid antibodies in people with Hashimoto’s were reduced by 40 percent in three to six months, with therapeutic doses of selenium. [91] This effect has been shown to be enhanced by the supplementation of myo-inositol alongside selenium, which I mentioned earlier. Research has shown that a combination of selenium and myo-inositol, at a daily dose of 83 mcg of selenium and 600 mg of myo-inositol, can have a synergistic effect that maximizes the benefit to thyroid function — consuming both of these together may not just reduce thyroid antibodies, but may also reduce TSH by an average of 30 percent. In some cases, this can normalize TSH levels (both for low and high TSH levels), reduce the risk of developing overt hypothyroidism, and improve overall well-being for those who have Hashimoto’s. [92]
When it comes to OCD, research has shown that selenium is an effective adjunct therapy to SSRI medications. In a study with 32 patients with a treatment-resistant obsessive-compulsive disorder, the intervention group received a daily 200 mcg selenium capsule, and each daily control group received one capsule of placebo for six weeks. [93] At the end of the study, Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores decreased in both groups, but this reduction was significantly lower in the selenium group.
Because of the synergistic effects of selenium and myo-inositol, I formulated Rootcology Selenium + Myo-Inositol, which delivers the therapeutic range of both selenium and myo-inositol in one supplement. (For optimal absorption, consider taking selenium and vitamin E together with food.)
In my survey, 63 percent of respondents said taking selenium made them feel better. See the precautions relating to selenium at the linked article.
- Amino Acids: A variety of amino acids are needed to support neurotransmitter synthesis and gut health, and may be helpful in addressing OCD symptoms. We get our amino acids from eating protein, so many people with Hashimoto’s may be deficient due to low stomach acid or issues relating to poor protein digestion. Animal protein is a better source of amino acids, so those who eat vegan or vegetarian diets can become deficient. There is advanced amino acid testing available to see what specific deficiencies you have. See the linked article for testing and other information on amino acids (including a description of what various ones do for the body). Here are a few that are of particular benefit in OCD:
- N-Acetyl Cysteine (NAC) is actually a supplement made from the amino acid cysteine. NAC has been found to regulate (and inhibit) excessive levels of glutamate, and result in a significant reduction in OCD symptoms. [94] Multiple animal studies have shown that NAC can reduce OCD-like behaviors, and several case reports have found that NAC can treat OCD. [95] Other randomized control trials found that NAC significantly reduced Yale-Brown Obsessive Compulsive Scale (YBOCS) scores compared to placebo.
- It also has benefits for those with Hashimoto’s! Research has found it helps reduce thyroid antibodies, it supports detoxification pathways, has antioxidant effects and anti-inflammatory properties, and I often recommend it to help address leaky gut and to improve gut function. [96] NAC has also been found to address Streptococcus bacteria by lowering its adhesion ability on the mucosal layer of the gut. [97] You can read much more about NAC at the linked article. If you think NAC might be a good option for you, consider Rootcology’s Pure N-Acetyl Cysteine Daily doses of 1,800 mg are usually recommended.
- Taurine is a precursor to GABA (which helps maintain healthy serotonin levels and reuptake), and has been found to reverse elevated glutamate in rat studies. [98] It has a calming effect (much like GABA), and has also been shown to have beneficial effects against a number of neurological disorders and be protective against toxicities of the nervous system. [99]
- N-Acetyl Cysteine (NAC) is actually a supplement made from the amino acid cysteine. NAC has been found to regulate (and inhibit) excessive levels of glutamate, and result in a significant reduction in OCD symptoms. [94] Multiple animal studies have shown that NAC can reduce OCD-like behaviors, and several case reports have found that NAC can treat OCD. [95] Other randomized control trials found that NAC significantly reduced Yale-Brown Obsessive Compulsive Scale (YBOCS) scores compared to placebo.
- Serotonin Precursor Amino Acids: While potentially very relevant in OCD, please note that I generally don’t like recommending serotonin precursor amino acids without oversight from a medical professional that is trained in using them (some people may find they become more agitated when taking them, as one example). There are two to consider for OCD.
- Tryptophan in particular is very important in the synthesis of serotonin. Its metabolism is also a key modulator of the gut microbiota, and protective of the intestinal barrier (which prevents leaky gut). [100]
- 5-HTP (5-hydroxytryptophan) is another precursor to serotonin. There are several studies looking at the beneficial use of 5-HTP in the treatment of OCD and other neurological conditions related to serotonin. [101] I often recommend 5-HTP for clients having trouble with sleep, as serotonin converts to melatonin. Please note that 5-HTP should not be taken with SSRI medications without doctor supervision.
Balance Your Gut
I talked previously about the importance of gut health due to the “gut-brain” axis and health of the gut microbiome. Please review this article on the importance of gut health in Hashimoto’s to learn more about my specific recommendations (there are many supportive nutrients and lifestyle interventions to heal and optimize the gut).
We know that having some level of leaky gut is one of the three requirements for developing an autoimmune condition (the other two being a genetic predisposition and one or more triggers), so this should be a key area of your focus for addressing Hashimoto’s, as well as addressing OCD symptoms.
The linked article will provide many core recommendations, but resolving Strep infections in the gut is one important protocol I want to focus on here, given its strong link to OCD.
You can test your microbiome to see if you have an overgrowth of Strep with a test like the GI-MAP.
Strep Protocols
- Black Seed Oil has been shown in multiple laboratory studies to inhibit the growth of a variety of Strep strains, including Streptococcus pyogene, as well as other infections and viruses. [102] The active ingredient of black seed oil is thymoquinone, which is anti-inflammatory, antiviral, antimicrobial, immunomodulatory, and gastro-protective, as well as possessing antioxidant properties. [103] Black seed oil may improve thyroid health, help clients stabilize their blood sugar, and help to address a number of gut infections. [104]
If you’re looking for a high-quality option, consider Rootcology’s Black Cumin Seed Extract, which is combined with vitamin E for a synergistic effect to boost its anti-inflammatory properties. (Please note that you should not take this product if you’re taking any of the following medications: alkylating agents, anticoagulant/antiplatelet drugs, antitumor antibiotics, blood thinners, cyclosporine, cytochrome P450 substrates (3A4), and/or selumetinib). Other options include a cold-pressed liquid form like this one from ZHOU Nutrition (although I will say it’s not the most pleasant tasting). It’s available on Fullscript – if you don’t have a Fullscript account, you can sign up with my credentials here. It also comes as a powder like this one from Amazing Herbs. The most common therapeutic dose in studies was 2 grams per day.
- Allicin (from garlic) has been shown to inhibit a key virulence factor of Streptococci (SpeB). [105] It has been studied for its ability to eradicate Streptococcus agalactiae in pregnant women, as this infection can be passed to the baby during birth and cause serious complications, including death. In folk medicine, garlic has been used to treat this infection. In more recent times, antibiotics have been used, but there is growing concern about antibiotic resistance. In laboratory settings, studies have shown that allicin can treat Streptococcus agalactiae and other strains of Strep. [106] Consider Rootcology’s Garlic Oil.
- Berberine has been found to exhibit antibacterial activity against Streptococcus and other infections. [107] Similar to NAC, berberine appears to interfere with the adherence of Streptococci on the mucosa. It also dissolves certain complexes of the bacteria. [108] In other research, it has been found to affect Streptococcus pyogenes via a variety of mechanisms. [109]
In a mouse study, berberine was found to reduce OCD symptoms (as defined in this study by marble burying behavior). The researchers determined that the effects of the berberine on OCD symptoms may be attributed to its effect on neurotransmitter systems, and not increased serotonin turnover in the brain. However, they were unable to determine the exact mechanism. [110] I wonder if perhaps the reason could be related to unknown Strep bacteria in the mice that berberine would have resolved?
I also often recommend berberine to help with lowering blood sugar levels (its effectiveness is similar to common diabetes medications!), which may improve OCD symptoms as well. Because of its multitasking benefits, I recently added Berberine to the Rootcology product line.
- St John’s Wort is an herb that has been used for a number of things (depression in particular), and was one of the first natural remedies I wrote about back in my Medical Chemistry class in high school! It has been shown to be effective for addressing Strep and Staph infections, depression, and (in one study) relieving OCD symptoms. [111] It contains an extract called Hypericum perforatum, which has been shown to be as effective as the SSRI fluoxetine, as well as several tricyclic antidepressants (in the treatment of depression). [112] It acts as a reuptake inhibitor (like SSRIs) for neurotransmitters. H. perforatum has shown antibacterial activity against many bacteria, including Streptococcus mutans and Streptococcus sobrinus, among others. [113] While the studies for using St. John’s wort for OCD are limited, I have seen some people anecdotally find significant relief with St. John’s wort. My personal belief is that this is due to its anti-Strep activity.
Please note that this herb can cause photosensitivity and has drug interactions with various drugs such as birth control, so it’s not usually my first choice. [114] There’s also some anecdotal evidence that it could increase TSH levels. [115] Be sure to discuss it with your doctor.
- Probiotics can be helpful for Strep overgrowth and OCD (as it balances the gut, which is in charge of producing neurotransmitters). You can eat fermented foods or supplement with the “right” probiotic (which is one that does not include Streptococcus, in most cases). However, if you have a history of recurrent Strep throat infections or want to be preventative in having them in the future, you may want to consider a beneficial strain of Streptococcal bacteria that can then overtake the pathogenic variety. One beneficial strain of S. salivarius, called K12, has been found in individuals who show a natural resistance to Streptococcus pyogenes. In research, K12 has been found to inhibit many strains of Streptococcus pyogenes and may help prevent strep throat. [116] They are daily lozenges. My son’s pediatrician told me about them and he did not have a strep infection until we forgot to take them. Last Christmas, I recommended them to a mom friend whose kids kept getting Strep infections — they have not gotten them since, and it’s been over six months. There is a K12 lozenge probiotic from Hyperbiotics which I’ve used with my son with great results! Another option are these ones from OralProbio.
Other Supportive Nutrients that May Help With OCD
- Milk thistle contains silymarin which, at a dose of 600 mg per day, was shown to exhibit the same improvements to OCD as fluoxetine, an SSRI. [117] Silymarin has antioxidant, antidepressant, anti-inflammatory and immune-modulating effects, and has been found to increase serotonin in the cortex. [118] Milk thistle is also very supportive of the liver (which we know can be congested in Hashimoto’s).
- Turmeric contains curcumin, another nutrient that is supportive of the liver. In one animal study, curcumin was shown to improve OCD symptoms. The study found improvements at both 5 and 10 mg/kg of body weight per day. Another animal study showed it to be effective in treating PTSD. [119]
- Vitamin D is vital to antioxidant processes, immunity modulation, and our inflammatory response. [120] It also plays a role in several nervous system processes, including neurotransmission and neuroprotection — so deficiency may affect serotonin as well as overall neuro-inflammation. Studies have demonstrated its deficiency to be associated with several neuropsychiatric conditions, including depression, autism, schizophrenia, and OCD. [121] There are two available tests to check your vitamin D levels: 1,25 (OH)D (which checks for the active form of vitamin D) and 25(OH)D (which checks for both D2 and D3). The 25(OH)D (25-hydroxyvitamin D) test is preferred, as it tests for both vitamin D2 and D3 levels and more accurately reflects one’s vitamin D status.
Overview of OCD Protocol
This article outlined a lot of information pertaining to OCD, so I thought it might be helpful to have a condensed protocol of my recommendations on how to approach OCD from the root cause perspective, at least as a starting point.
If I had a client, friend, or family member presenting with OCD, I would recommend the following to start.
Testing
Testing for the following will help identify deficiencies or infections that may be contributing to OCD symptoms.
- Vitamin B12: To find out if you have low levels of B12, you can ask your healthcare provider for the B12 (or cobalamin) test. Alternatively, you can self-order the B12 test via Ulta Lab Tests. When you receive your test results, it’s important to note that optimal B12 levels should be between 700 and 900 pg/mL. As mentioned above, most labs will not flag B12 levels unless they are under 200 pg/mL. Please note that testing may not always be reliable, as the consumption of bread and cereals fortified with folic acid (synthetic folate) may mask a B12 deficiency on standard lab tests.
- Vitamin D: There are two available tests to check your vitamin D levels: 1,25 (OH)D (which checks for the active form of vitamin D) and 25(OH)D (which checks for both D2 and D3). The 25(OH)D (25-hydroxyvitamin D) test is preferred, as it tests for both vitamin D2 and D3 levels and more accurately reflects one’s vitamin D status. Optimal levels are between 60 and 80 ng/mL.
- Homocysteine: Testing for homocysteine levels is now available through many labs and can be assessed with a blood test. According to functional ranges, the optimal homocysteine levels range is somewhere between 5 and 7 µmol/L. As for testing for the MTHFR genetic variation, many labs also offer tests for this gene. For example, you can order a genetic saliva test kit from 23andme.com, or see if you can get a genetic test from your physician, which may be covered by insurance. You can upload your results to geneticgenie.org, which will then tell you if you have the genetic variation.
- GI-MAP: Consider gut testing to see if you have an overgrowth of Strep in the gut.
Interventions
I would then encourage my loved ones to investigate one or more of the following approaches, which are generally well-tolerated.
- Low glutamate diet + P5P: Medications that modulate glutamate have been shown to be effective in treating OCD in some people, and in one case study, restricting the intake of free glutamate resolved the patient’s treatment-resistant OCD. Foods containing high levels of free glutamate include: bone broth, some soy-containing Asian sauces, aged cheeses like parmesan, and many types of flavor-enhancing food additives such as MSG, mushrooms, and tomato sauce. Additionally, consider a P5P supplement, as a deficiency in vitamin B6 can result in excess conversion of glutamine to glutamate. Rootcology P5P or Pure Encapsulations P5P 50 are both high-quality options. Consider 50 mg per day.
- NAC: NAC is also helpful for excessive levels of glutamate, and can result in a significant reduction in OCD symptoms. NAC has also been found to address Streptococcus bacteria by lowering its adhesion ability on the mucosal layer of the gut. If you are looking for a high-quality option, consider Rootcology’s Pure N-Acetyl Cysteine or Pure Encapsulations NAC. Daily doses of 1,800 mg are usually recommended.
- Myo-Inositol: Myo-inositol has been well studied for its positive effects on anxiety and OCD. Studies have used 18 g of myo-inositol per day, but start with 3 g per day and work your way up. Many people feel benefits at 12 g or less per day. Rootcology makes a Myo-Inositol Powder that is easy to take (P.S. get 10% off with code MYOINOSITOL10 now through January 17th at 11:59 pm PT), as does Pure Encapsulations. Note: If you have kidney disease or are taking diuretic medications, please check with your practitioner before taking myo-inositol.
- Selenium: Research has shown that selenium is an effective adjunct therapy to SSRI medications for OCD. It can also help lower thyroid antibodies, which can be helpful for those whose OCD is related to elevated antibodies. Research suggests that 200 mcg per day can be helpful, but if combined with 600 g of myo-inositol, a dose of 83 mcg is sufficient. Rootcology’s Selenium + Myo-Inositol delivers the therapeutic range of both nutrients in one supplement. If you’d prefer a standalone selenium supplement, then you may wish to consider Pure Encapsulations Selenium.
- Berberine: Due to its benefits to blood sugar, neurotransmitters, and preventing Streptococcus from adhering to the gut, berberine is another option that may be helpful for OCD. A dose of 400 to 500 mg three times per day is the typical dose used in most studies, though a great place to start is 400 to 500 mg once per day. A few high-quality options include Rootcology Berberine, Pure Encapsulations Berberine, and Designs for Health Berberine. Please note that those with Crohn’s, colitis, or ulcers should be cautious when using berberine, and I suggest working under a doctor’s supervision.
- Milk Thistle: Milk thistle has been shown to be just as effective as a common SSRI for OCD symptoms, at a dose of 600 mg per day. Consider Pure Encapsulations Silymarin or Design for Health’s Milk Thistle.
- Turmeric: Curcumin, the active ingredient in turmeric, has been shown to be helpful for OCD symptoms as well. Consider either Rootcology’s Curcumin Absorb or Thorne’s Curcumin Phytosome (available via Fullscript – if you don’t have a Fullscript account, you can sign up with my credentials here). Doses used in research vary from 5 to 10 mg/kg of body weight per day, but doses between 500 and 2,000 mg per day are generally considered safe.
- B12: Some researchers have speculated that because of vitamin B12’s role in neurotransmitter synthesis, OCD can sometimes be an early manifestation of vitamin B12 deficiency. High-quality B12 supplements include Pure Encapsulations Adenosyl/Hydroxy B12, liquid Pure Encapsulations B12, and Designs for Health B12 Lozenges. Sublingual doses of 5 mg (5000 mcg) of B12, daily for ten days, then 5 mg once per week for four weeks, then 5 mg monthly, have been found to be effective in restoring B12 levels in those with a deficiency.
There is also some evidence that 5-HTP and GABA can be helpful for OCD. You can read more about the research behind them here and here. While I have found them to be helpful for clients, in general, I advise working with a health professional who has experience with them, as 5-HTP can worsen symptoms in some, and GABA can over-convert to glutamate if you’re deficient in P5P.
I also love the books This Is Your Brain on Food: An Indispensable Guide to the Surprising Foods that Fight Depression, Anxiety, PTSD, OCD, ADHD, and More by Uma Naidoo, and The Antianxiety Food Solution: How the Foods You Eat Can Help You Calm Your Anxious Mind, Improve Your Mood, and End Cravings by Trudy Scott, as resources.
And of course, I recommend working with your doctor and consulting with them as you implement lifestyle changes. Please note that if you are currently taking prescription medications to manage your OCD, it is important not to stop taking your medications without the oversight of your physician or therapist.
Takeaway
Going back to my lucid dream that I mentioned at the beginning of this article… the next time I dreamt, I did things differently during that doctor’s appointment! When he suggested antidepressants, I stopped him mid-sentence and said, “No, Doctor, I don’t need antidepressants. I came in because of my physical symptoms.” I may have thrown a swear word or two in there somewhere. And it was liberating!
Now that I’m a rebel with a cause — a Root Cause Rebel — my voice is getting stronger, and my pathological politeness is getting weaker each and every day.
So if you’ve ever been told that it’s all in your head (your physical or mental symptoms!), or that you need antidepressants, or that you should just settle for the status quo, say it with me, my fellow Root Cause Rebel: “No, Doctor, I don’t need antidepressants!”
Then get to work on addressing your root causes. There is a lot of good information here for you to work with on your own or with a good functional doctor. But don’t let the information overwhelm you. I have found that it is easiest to tackle one intervention at a time. A good starting point is to ensure your thyroid meds and hormone levels are optimized, and to ensure you know your thyroid antibody numbers. Then a few key nutrients can really help. As your thyroid antibody counts drop, so should some of those obsessive-compulsive tendencies.
As always, let me know how you do, and feel free to ask my team questions.
P.S. I love interacting with my readers on social media, and I encourage you to join my Facebook, Instagram, TikTok, and Pinterest community pages to stay on top of thyroid health updates and meet others who are following similar health journeys. For recipes, a FREE Thyroid Diet start guide, notifications about upcoming events, and the Nutrient Depletions and Digestion chapter from my first book for free, be sure to sign up for my email list!
References
[1] Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype?. Transl Psychiatry. 2022;12(1):5. Published 2022 Jan 10. doi:10.1038/s41398-021-01700-4
[2] National Collaborating Centre for Mental Health (UK). Obsessive-Compulsive Disorder: Core Interventions in the Treatment of Obsessive-Compulsive Disorder and Body Dysmorphic Disorder. Leicester (UK): British Psychological Society (UK); 2006. (NICE Clinical Guidelines, No. 31.) Appendix 15, Diagnostic criteria. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56452/; Bralten J, Widomska J, Witte W, et al. Shared genetic etiology between obsessive-compulsive disorder, obsessive-compulsive symptoms in the population, and insulin signaling. Transl Psychiatry. 2020;10(1):121. Published 2020 Apr 27. doi:10.1038/s41398-020-0793-y
[3] Janardhan Reddy YC, Sundar AS, Narayanaswamy JC, et al. Clinical practice guidelines for Obsessive-Compulsive Disorder. Indian J Psychiatry. 2017;59(Suppl 1):S74-S90. doi:10.4103/0019-5545.196976
[4] Brock H, Hany M. Obsessive-Compulsive Disorder. [Updated 2022 Feb 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553162/
[5] Franklin ME, Harrison JP, Benavides KL. Obsessive-compulsive and tic-related disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):555-571. doi:10.1016/j.chc.2012.05.008
[6] Jain S, Hirst RB, Chu DJ, et al. Establishing a biological psychiatric diagnostic classification using inflammatory markers and large-scale machine learning. Transl Psychiatry. 2020;10(1):1-12. doi:10.1038/s41398-020-0793-y
[7] Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype?. Transl Psychiatry. 2022;12(1):5. Published 2022 Jan 10. doi:10.1038/s41398-021-01700-4
[8] Attwells S, Setiawan E, Wilson AA, et al. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry. 2017;74(8):833-840; Çayköylü A, Uğurlu GK, Yenilmez DO, Çayköylü HH, Uğurlu M. Subthreshold Obsessive-Compulsive Symptoms in 3 Patients With Papillary Thyroid Carcinoma. Prim Care Companion CNS Disord. 2020;22(1):19l02463. Published 2020 Jan 23. doi:10.4088/PCC.19l02463
[9] Bralten J, Widomska J, Witte W, et al. Shared genetic etiology between obsessive-compulsive disorder, obsessive-compulsive symptoms in the population, and insulin signaling. Transl Psychiatry. 2020;10(1):121. Published 2020 Apr 27. doi:10.1038/s41398-020-0793-y
[10] Savaheli S, Ahmadiani A. Obsessive-compulsive disorder and growth factors: A comparative review. Behav Brain Res. 2019 Oct 17;372:111967. doi: 10.1016/j.bbr.2019.111967. Epub 2019 May 25. PMID: 31136772; Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype?. Transl Psychiatry. 2022;12(1):5. Published 2022 Jan 10. doi:10.1038/s41398-021-01700-4
[11] Brock H, Hany M. Obsessive-Compulsive Disorder. [Updated 2022 Feb 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553162/
[12] Burton CL, Park LS, Corfield EC, et al. Heritability of obsessive-compulsive trait dimensions in youth from the general population. Transl Psychiatry. 2018;8(1):191. Published 2018 Sep 18. doi:10.1038/s41398-018-0249-9
[13] Brock H, Hany M. Obsessive-Compulsive Disorder. [Updated 2022 Feb 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553162/
[14] Bralten J, Widomska J, Witte W, et al. Shared genetic etiology between obsessive-compulsive disorder, obsessive-compulsive symptoms in the population, and insulin signaling. Transl Psychiatry. 2020;10(1):121. Published 2020 Apr 27. doi:10.1038/s41398-020-0793-y; Savaheli S, Ahmadiani A. Obsessive-compulsive disorder and growth factors: A comparative review. Behav Brain Res. 2019 Oct 17;372:111967. doi: 10.1016/j.bbr.2019.111967. Epub 2019 May 25. PMID: 31136772.
[15] Brock H, Hany M. Obsessive-Compulsive Disorder. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 29, 2023.
[16]Brock H, Hany M. Obsessive-Compulsive Disorder. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 29, 2023.; Bralten J, Widomska J, Witte W, et al. Shared genetic etiology between obsessive-compulsive disorder, obsessive-compulsive symptoms in the population, and insulin signaling. Transl Psychiatry. 2020;10(1):121. Published 2020 Apr 27. doi:10.1038/s41398-020-0793-y
[17] Real E, Labad J, Alonso P, et al. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress Anxiety. 2011;28(5):367-376. doi:10.1002/da.20792
[18] Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr Psychiatry Rep. 2019;21(8):78. Published 2019 Aug 1. doi:10.1007/s11920-019-1062-8; Vallée A, Vallée JN, Lecarpentier Y. Lithium: a potential therapeutic strategy in obsessive-compulsive disorder by targeting the canonical WNT/β pathway. Transl Psychiatry. 2021;11(1):204. Published 2021 Apr 7. doi:10.1038/s41398-021-01329-3
[19] Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype?. Transl Psychiatry. 2022;12(1):5. Published 2022 Jan 10. doi:10.1038/s41398-021-01700-4; Arnold PD, Richter MA. Is obsessive-compulsive disorder an autoimmune disease?. CMAJ. 2001;165(10):1353-1358.
[20] Della Vecchia A, Marazziti D. Back to the Future: The Role of Infections in Psychopathology. Focus on OCD. Clin Neuropsychiatry. 2022;19(4):248-263. doi:10.36131/cnfioritieditore20220407
[21] Ashfaq-U-Rahaman, Janardhan Reddy YC, Prabhavathi, Pal PK. Obsessive compulsive disorder in adults with rheumatic heart disease. Acta Neuropsychiatr. 2007;19(2):118-121. doi:10.1111/j.1601-5215.2006.00169.x
[22] Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME. Association of Streptococcal Throat Infection With Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry. 2017;74(7):740-746. doi:10.1001/jamapsychiatry.2017.0995
[23] Bodner SM, Morshed SA, Peterson BS. The question of PANDAS in adults. Biol Psychiatry. 2001;49(9):807-810. doi:10.1016/s0006-3223(00)01127-6
[24] Wang HC, Lau CI, Lin CC, Chang A, Kao CH. Group A Streptococcal Infections Are Associated With Increased Risk of Pediatric Neuropsychiatric Disorders: A Taiwanese Population-Based Cohort Study. J Clin Psychiatry. 2016;77(7):e848-e854. doi:10.4088/JCP.14m09728
[25] Attwells S, Setiawan E, Wilson AA, et al. Inflammation in the Neurocircuitry of Obsessive-Compulsive Disorder. JAMA Psychiatry. 2017 Aug 1;74(8):833-840. doi: 10.1001/jamapsychiatry.2017.1567. PMID: 28636705; PMCID: PMC5710556.
[26] Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr Psychiatry Rep. 2019;21(8):78. Published 2019 Aug 1. doi:10.1007/s11920-019-1062-8
[27] Real E, Labad J, Alonso P, et al. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress Anxiety. 2011;28(5):367-376. doi:10.1002/da.20792
[28] Pittenger C. Pharmacotherapeutic Strategies and New Targets in OCD. Curr Top Behav Neurosci. 2021;49:331-384. doi:10.1007/7854_2020_204
[29] Real E, Labad J, Alonso P, et al. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress Anxiety. 2011;28(5):367-376. doi:10.1002/da.20792
[30] Goodman WK, Storch EA, Sheth SA. Harmonizing the Neurobiology and Treatment of Obsessive-Compulsive Disorder. Am J Psychiatry. 2021;178(1):17-29. doi:10.1176/appi.ajp.2020.20111601
[31] Cope EC, Levenson CW. Role of zinc in the development and treatment of mood disorders. Curr Opin Clin Nutr Metab Care. 2010;13(6):685-689. doi:10.1097/MCO.0b013e32833df61a; Rajizadeh A, Mozaffari-Khosravi H, Yassini-Ardakani M, Dehghani A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition. 2017;35:56-60. doi:10.1016/j.nut.2016.10.014; Field DT, Cracknell RO, Eastwood JR, et al. High-dose Vitamin B6 supplementation reduces anxiety and strengthens visual surround suppression. Hum Psychopharmacol. 2022;37(6):e2852. doi:10.1002/hup.2852; Külz AK, Meinzer S, Kopasz M, Voderholzer U. Effects of tryptophan depletion on cognitive functioning, obsessive-compulsive symptoms and mood in obsessive-compulsive disorder: preliminary results. Neuropsychobiology. 2007;56(2-3):127-131. doi:10.1159/000115778
[32] Real E, Labad J, Alonso P, et al. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress Anxiety. 2011;28(5):367-376. doi:10.1002/da.20792
[33] Karpinski M, Mattina GF, Steiner M. Effect of Gonadal Hormones on Neurotransmitters Implicated in the Pathophysiology of Obsessive-Compulsive Disorder: A Critical Review. Neuroendocrinology. 2017;105(1):1-16. doi:10.1159/000453664
[34] Karthik S, Sharma LP, Narayanaswamy JC. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr Dis Treat. 2020;16:1003-1013. Published 2020 Apr 17. doi:10.2147/NDT.S211703; Vallée A, Vallée JN, Lecarpentier Y. Lithium: a potential therapeutic strategy in obsessive-compulsive disorder by targeting the canonical WNT/β pathway. Transl Psychiatry. 2021;11(1):204. Published 2021 Apr 7. doi:10.1038/s41398-021-01329-3
[35] Karthik S, Sharma LP, Narayanaswamy JC. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr Dis Treat. 2020;16:1003-1013. Published 2020 Apr 17. doi:10.2147/NDT.S211703
[36] Vallée A, Vallée JN, Lecarpentier Y. Lithium: a potential therapeutic strategy in obsessive-compulsive disorder by targeting the canonical WNT/β pathway. Transl Psychiatry. 2021;11(1):204. Published 2021 Apr 7. doi:10.1038/s41398-021-01329-3; Hadi F, Kashefinejad S, Kamalzadeh L, et al. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2021 Nov 4;22(1):69. doi: 10.1186/s40360-021-00534-6. PMID: 34736541; PMCID: PMC8569963; Vallée A, Vallée JN, Lecarpentier Y. Lithium: a potential therapeutic strategy in obsessive-compulsive disorder by targeting the canonical WNT/β pathway. Transl Psychiatry. 2021;11(1):204. Published 2021 Apr 7. doi:10.1038/s41398-021-01329-3; Gerentes M, Pelissolo A, Rajagopal K, et al. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr Psychiatry Rep. 2019 Aug 1;21(8):78. doi: 10.1007/s11920-019-1062-8. PMID: 31367805.
[37] Karthik S, Sharma LP, Narayanaswamy JC. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr Dis Treat. 2020;16:1003-1013. Published 2020 Apr 17. doi:10.2147/NDT.S211703
[38] Hansen AM, Caspi RR. Glutamate joins the ranks of immunomodulators. Nat Med. 2010;16(8):856-858. doi:10.1038/nm0810-856
[39] Marinova Z, Chuang DM, Fineberg N. Glutamate-Modulating Drugs as a Potential Therapeutic Strategy in Obsessive-Compulsive Disorder. Curr Neuropharmacol. 2017;15(7):977-995. doi:10.2174/1570159X15666170320104237
[40] Calderón-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. 2020;26(1):5-13. doi:10.1111/cns.13207
[41] Mesripour A, Hajhashemi V, Kuchak A. Effect of concomitant administration of three different antidepressants with vitamin B6 on depression and obsessive compulsive disorder in mice models. Res Pharm Sci. 2017;12(1):46-52. doi:10.4103/1735-5362.199046
[42] Marazziti D, Buccianelli B, Palermo S, et al. The Microbiota/Microbiome and the Gut-Brain Axis: How Much Do They Matter in Psychiatry?. Life (Basel). 2021;11(8):760. Published 2021 Jul 28. doi:10.3390/life11080760; O’Mahony SM, Clarke G, Borre YE, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015 Jan 15;277:32-48. doi: 10.1016/j.bbr.2014.07.027. Epub 2014 Jul 29. PMID: 25078296; Jenkins TA, Nguyen JC, Polglaze KE, et al. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8(1):56. Published 2016 Jan 20. doi:10.3390/nu8010056; Marazziti D, Buccianelli B, Palermo S, et al. The Microbiota/Microbiome and the Gut-Brain Axis: How Much Do They Matter in Psychiatry?. Life (Basel). 2021;11(8):760. Published 2021 Jul 28. doi:10.3390/life11080760
[43] Maia A, Oliveira J, Lajnef M, et al. Oxidative and nitrosative stress markers in obsessive-compulsive disorder: a systematic review and meta-analysis. Acta Psychiatr Scand. 2019;139(5):420-433. doi:10.1111/acps.13026; Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. 2014;8(12):CC04-CC7. doi:10.7860/JCDR/2014/10258.5292
[44] Kandemir H, Abuhandan M, Aksoy N, et al. Oxidative imbalance in child and adolescent patients with obsessive compulsive disorder. J Psychiatr Res. 2013 Nov;47(11):1831-4. doi: 10.1016/j.jpsychires.2013.08.010. Epub 2013 Aug 22. PMID: 24011862; Mancini A, Di Segni C, Raimondo S, et al. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediators Inflamm. 2016;2016:6757154. doi:10.1155/2016/6757154; Behl A, Swami G, Sircar SS, et al. Relationship of possible stress-related biochemical markers to oxidative/antioxidative status in obsessive-compulsive disorder. Neuropsychobiology. 2010;61(4):210-4. doi: 10.1159/000306591. Epub 2010 Apr 8. PMID: 20389131; Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24(5):725-743. doi:10.1016/j.jnutbio.2013.01.002.
[45] Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54. doi:10.1146/annurev.med.60.041807.123308
[46] Yan S, Liu H, Yu Y, Han N, Du W. Changes of Serum Homocysteine and Vitamin B12, but Not Folate Are Correlated With Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis of Case-Control Studies. Front Psychiatry. 2022;13:754165. Published 2022 May 9. doi:10.3389/fpsyt.2022.754165; Balandeh E, Karimian M, Behjati M, Mohammadi AH. Serum Vitamins and Homocysteine Levels in Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis. Neuropsychobiology. 2021;80(6):502-515. doi:10.1159/000514075
[47] Gilfix BM. Vitamin B12 and homocysteine. CMAJ. 2005;173(11):1360. doi:10.1503/cmaj.1050170
[48] Arakawa Y, Watanabe M, Inoue N, et al. Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clinical and Experimental Immunology. 2012;170(2):194–201. doi:10.1111/j.1365-2249.2012.04646.; Kvaratskhelia T, Abzianidze E, Asatiani K, Kvintradze M, Surmava S, Kvaratskhelia E. Methylenetetrahydrofolate Reductase (MTHFR) C677T and A1298C Polymorphisms in Georgian Females with Hypothyroidism. Glob Med Genet. 2020;7(2):47-50. doi:10.1055/s-0040-1714091
[49] Witthauer C, T Gloster A, Meyer AH, et al. Physical diseases among persons with obsessive compulsive symptoms and disorder: a general population study. Soc Psychiatry Psychiatr Epidemiol. 2014 Dec;49(12):2013-22. doi: 10.1007/s00127-014-0895-z. Epub 2014 Jun 8. PMID: 24907897; PMCID: PMC4228109; Çayköylü A, Uğurlu GK, Yenilmez DO, Çayköylü HH, Uğurlu M. Subthreshold Obsessive-Compulsive Symptoms in 3 Patients With Papillary Thyroid Carcinoma. Prim Care Companion CNS Disord. 2020;22(1):19l02463. Published 2020 Jan 23. doi:10.4088/PCC.19l02463
[50] Caykoylu A, Kabadayi Sahin E, Ugurlu M. Could the Thyroid Gland Dominate the Brain in Obsessive-Compulsive Disorder?. Neuroendocrinology. 2022;112(12):1143-1154. doi:10.1159/000524627
[51] Cai YJ, Wang F, Chen ZX, et al. Hashimoto’s thyroiditis induces neuroinflammation and emotional alterations in euthyroid mice. J Neuroinflammation. 2018 Oct 29;15(1):299. doi: 10.1186/s12974-018-1341-z. PMID: 30373627; PMCID: PMC6206655; Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7(2):140-56. doi: 10.1038/sj.mp.4000963. PMID: 11840307
[52] Wu, X, Zhang, K, Xing, Y, et al. Dysregulated thyroid hormones correlate with anxiety and depression risk in patients with autoimmune disease. J Clin Lab Anal. 2021; 35:e23573. https://doi.org/10.1002/jcla.23573
[53] Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226-238. doi:10.1016/j.pharmthera.2011.01.014; Çayköylü A, Uğurlu GK, Yenilmez DO, Çayköylü HH, Uğurlu M. Subthreshold Obsessive-Compulsive Symptoms in 3 Patients With Papillary Thyroid Carcinoma. Prim Care Companion CNS Disord. 2020;22(1):19l02463. Published 2020 Jan 23. doi:10.4088/PCC.19l02463; Cai YJ, Wang F, Chen ZX, et al. Hashimoto’s thyroiditis induces neuroinflammation and emotional alterations in euthyroid mice. J Neuroinflammation. 2018 Oct 29;15(1):299. doi: 10.1186/s12974-018-1341-z. PMID: 30373627; PMCID: PMC6206655.
[54] Wang N, Sun Y, Yang H, et al. Hashimoto’s Thyroiditis Induces Hippocampus-Dependent Cognitive Alterations by Impairing Astrocytes in Euthyroid Mice. Thyroid. 2021;31(3):482-493. doi:10.1089/thy.2020.0139
[55] Blanchin S, Coffin C, Viader F, et al. Anti-thyroperoxidase antibodies from patients with Hashimoto’s encephalopathy bind to cerebellar astrocytes. J Neuroimmunol. 2007;192(1-2):13-20. doi:10.1016/j.jneuroim.2007.08.012
[56] Leyhe T, Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav Immun. 2014 Oct;41:261-6. doi: 10.1016/j.bbi.2014.03.008. Epub 2014 Mar 29. PMID: 24685840; Çayköylü A, Uğurlu GK, Yenilmez DO, Çayköylü HH, Uğurlu M. Subthreshold Obsessive-Compulsive Symptoms in 3 Patients With Papillary Thyroid Carcinoma. Prim Care Companion CNS Disord. 2020;22(1):19l02463. Published 2020 Jan 23. doi:10.4088/PCC.19l02463
[57] Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype?. Transl Psychiatry. 2022;12(1):5. Published 2022 Jan 10. doi:10.1038/s41398-021-01700-4; Wang LY, Chen SF, Chiang JH, et al. Systemic autoimmune diseases are associated with an increased risk of obsessive-compulsive disorder: a nationwide population-based cohort study. Soc Psychiatry Psychiatr Epidemiol. 2019 Apr;54(4):507-516. doi: 10.1007/s00127-018-1622-y. Epub 2018 Nov 7. PMID: 30406283.
[58] Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr Psychiatry Rep. 2019;21(8):78. Published 2019 Aug 1. doi:10.1007/s11920-019-1062-8
[59] Carta MG, Loviselli A, Hardoy MC, et al. The link between thyroid autoimmunity (antithyroid peroxidase autoantibodies) with anxiety and mood disorders in the community: a field of interest for public health in the future. BMC Psychiatry. 2004 Aug 18;4:25. doi: 10.1186/1471-244X-4-25. PMID: 15317653; PMCID: PMC516779
[60] Müssig K, Künle A, Säuberlich AL, et al. Thyroid peroxidase antibody positivity is associated with symptomatic distress in patients with Hashimoto’s thyroiditis. Brain Behav Immun. 2012;26(4):559-563. doi:10.1016/j.bbi.2012.01.006
[61] Leyhe T, Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav Immun. 2014;41:261-266. doi:10.1016/j.bbi.2014.03.008.
[62] Vogel L. Growing consensus on link between strep and obsessive-compulsive disorder. CMAJ. 2018;190(3):E86-E87. doi:10.1503/cmaj.109-5545
[63] Wu BG, Sulaiman I, Wang J, et al. Severe Obstructive Sleep Apnea Is Associated with Alterations in the Nasal Microbiome and an Increase in Inflammation. Am J Respir Crit Care Med. 2019;199(1):99-109. doi:10.1164/rccm.201801-0119OC; Bozkurt NC, Karbek B, Cakal E, Firat H, Ozbek M, Delibasi T. The association between severity of obstructive sleep apnea and prevalence of Hashimoto’s thyroiditis. Endocr J. 2012;59(11):981-988. doi:10.1507/endocrj.ej12-0106
[64] Arnold PD, Richter MA. Is obsessive-compulsive disorder an autoimmune disease?. CMAJ. 2001;165(10):1353-1358.
[65] Carding S, Verbeke K, Vipond DT, et al. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. Published 2015 Feb 2. doi:10.3402/mehd.v26.26191
[66] Huang S, Zhou Y, Wu F, et al. Berberine Facilitates Extinction and Prevents the Return of Fear. Front Pharmacol. 2022;12:748995. Published 2022 Feb 3. doi:10.3389/fphar.2021.748995; Fan J, Zhang K, Jin Y, et al. Pharmacological effects of berberine on mood disorders. J Cell Mol Med. 2019;23(1):21-28. doi:10.1111/jcmm.13930
[67] Yue SJ, Liu J, Wang AT, et al. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am J Physiol Endocrinol Metab. 2019;316(1):E73-E85. doi:10.1152/ajpendo.00256.2018; van de Vondervoort IIGM, Amiri H, Bruchhage MMK, et al. Converging evidence points towards a role of insulin signaling in regulating compulsive behavior. Transl Psychiatry. 2019;9(1):225. Published 2019 Sep 12. doi:10.1038/s41398-019-0559-6
[68] Marazziti D, Buccianelli B, Palermo S, et al. The Microbiota/Microbiome and the Gut-Brain Axis: How Much Do They Matter in Psychiatry?. Life (Basel). 2021;11(8):760. Published 2021 Jul 28. doi:10.3390/life11080760
[69] Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261-1272. doi:10.1089/jmf.2014.7000
[70] Hadi F, Kashefinejad S, Kamalzadeh L, et al. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2021;22(1):69. Published 2021 Nov 4. doi:10.1186/s40360-021-00534-6
[71] Hadi F, Kashefinejad S, Kamalzadeh L, et al. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2021;22(1):69. Published 2021 Nov 4. doi:10.1186/s40360-021-00534-6; Karthik S, Sharma LP, Narayanaswamy JC. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr Dis Treat. 2020;16:1003-1013. Published 2020 Apr 17. doi:10.2147/NDT.S211703
[72] Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24(5):725-743. doi:10.1016/j.jnutbio.2013.01.002.
[73] Rodrigo L, Álvarez N, Fernández-Bustillo E, et al. Efficacy of a Gluten-Free Diet in the Gilles de la Tourette Syndrome: A Pilot Study. Nutrients. 2018 May 7;10(5):573. doi: 10.3390/nu10050573. PMID: 29735930; PMCID: PMC5986453.
[74] Holton KF, Cotter EW. Could dietary glutamate be contributing to the symptoms of obsessive-compulsive disorder? Future Sci OA. 2018 Jan 10;4(3):FSO277. doi: 10.4155/fsoa-2017-0105. PMID: 29568566; PMCID: PMC5859338
[75] Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159
[76] van de Vondervoort IIGM, Amiri H, Bruchhage MMK, et al. Converging evidence points towards a role of insulin signaling in regulating compulsive behavior. Transl Psychiatry. 2019;9(1):225. Published 2019 Sep 12. doi:10.1038/s41398-019-0559-6
[77] Danan A, Westman EC, Saslow LR, Ede G. The Ketogenic Diet for Refractory Mental Illness: A Retrospective Analysis of 31 Inpatients. Front Psychiatry. 2022;13:951376. Published 2022 Jul 6. doi:10.3389/fpsyt.2022.951376
[78] Zahiruddin S, Basist P, Parveen A, et al. Ashwagandha in brain disorders: A review of recent developments. J Ethnopharmacol. 2020 Jul 15;257:112876. doi: 10.1016/j.jep.2020.112876. Epub 2020 Apr 16. PMID: 32305638.
[79] Maxfield L, Shukla S, Crane JS. Zinc Deficiency. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 28, 2023.
[80]Piao, M et al. The Role of Zinc in Mood Disorders. Neuropsych. 2017 7(4): 378–386. doi:10.4172/NEUROPSYCHIATRY.1000225; Sayyah M, Olapour A, Saeedabad Ys, et al. Evaluation of oral zinc sulfate effect on obsessive-compulsive disorder: a randomized placebo-controlled clinical trial. Nutrition. 2012 Sep;28(9):892-5. doi: 10.1016/j.nut.2011.11.027. Epub 2012 Mar 30. PMID: 22465904; Botturi A, Ciappolino V, Delvecchio G, et al. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients. 2020;12(6):1661. Published 2020 Jun 3. doi:10.3390/nu12061661
[81] Sartori SB, Whittle N, Hetzenauer A, Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2012;62(1):304-312. doi:10.1016/j.neuropharm.2011.07.027
[82] Botturi A, Ciappolino V, Delvecchio G, et al. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients. 2020;12(6):1661. Published 2020 Jun 3. doi:10.3390/nu12061661
[83] Folkers K, Shizukuishi S, Willis R, Scudder SL, Takemura K, Longenecker JB. The biochemistry of vitamin B6 is basic to the cause of the Chinese restaurant syndrome. Hoppe Seylers Z Physiol Chem. 1984 Mar;365(3):405-14. doi: 10.1515/bchm2.1984.365.1.405. PMID: 6724532.
[84] Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159
[85] Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24(5):725-743. doi:10.1016/j.jnutbio.2013.01.002.
[86] Valizadeh M, Valizadeh N. Obsessive compulsive disorder as early manifestation of B12 deficiency. Indian J Psychol Med. 2011;33(2):203-204. doi:10.4103/0253-7176.92051
[87] Froes V, Afonso H, Gameiro Z. Obsessive-Compulsive Symptoms as a Manifestation of Homocystinuria. Case Rep Psychiatry. 2021;2021:5523453. Published 2021 Mar 20. doi:10.1155/2021/5523453; Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159
[88] Greenblatt, J. Integrative Therapies for Obsessive Compulsive Disorder. January 3, 2023. Mosaic Diagnostics. Accessed August 16, 2023. https://www.greatplainslaboratory.com/articles-1/2017/7/10 /integrative-therapies-for-obsessive-compulsive-disorder
[89] Levine J. Controlled trials of inositol in psychiatry. Eur Neuropsychopharmacol. 1997 May;7(2):147-55. doi: 10.1016/s0924-977x(97)00409-4. PMID: 9169302; Fux M, Benjamin J, Belmaker RH. Inositol versus placebo augmentation of serotonin reuptake inhibitors in the treatment of obsessive-compulsive disorder: a double-blind cross-over study. Int J Neuropsychopharmacol. 1999 Sep;2(3):193-195. doi: 10.1017/S1461145799001546. PMID: 11281989.
[90] Greenblatt J. Integrative Therapies for Obsessive Compulsive Disorder. Mosaic Diagnostics. January 3, 2023. Accessed October 11, 2023. https://mosaicdx.com/resource/integrative-therapies-for-obsessive -compulsive-disorder/.
[91] Mazokopakis EE, Papadakis JA, Papadomanolaki MG, et al. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO Levels in patients with Hashimoto’s thyroiditis. Thyroid. 2007 Jul;17(7):609-12.
[92] Nordio M, Basciani S. Evaluation of thyroid nodule characteristics in subclinical hypothyroid patients under a myo-inositol plus selenium treatment. Eur Rev Med Pharmacol Sci. 2018;22(7):2153-2159. doi:10.26355/eurrev_201804_14749; Nordio M, Pajalich R. Combined Treatment with Myo-Inositol and Selenium Ensures Euthyroidism in Subclinical Hypothyroidism Patients with Autoimmune Thyroiditis. Journal of Thyroid Research. 2013;2013:424163. doi:10.1155/2013/424163.; Nordio M, Basciani S. Treatment with Myo-Inositol and Selenium Ensures Euthyroidism in Patients with Autoimmune Thyroiditis. International Journal of Endocrinology. 2017;2017:2549491. doi:10.1155/2017/2549491.; Ferrari SM, Fallahi P, Di Bari F, Vita R, Benvenga S, Antonelli A. Myo-inositol and selenium reduce the risk of developing overt hypothyroidism in patients with autoimmune thyroiditis. Eur Rev Med Pharmacol Sci. 2017;21(2 Suppl):36-42.; Ferrari, SM et al. Precision Medicine in Autoimmune Thyroiditis and Hypothyroidism. Front. Pharmacol., 17 November 2021 | https://doi.org/10.3389/fphar.2021.750380
[93] Sayyah mehdi, Andishmand M, Ganji R. Effect of selenium as an adjunctive therapy in patients with treatment-resistant obsessive-compulsive disorder: A pilot randomized double blind placebo-controlled clinical trial. Archives of Psychiatry and Psychotherapy. December 12, 2018. Accessed October 11, 2023. https://www.archivespp.pl/Effect-of-selenium-as-an-adjunctive-therapy-nin-patients-with-treatment-resistant,99584,0,2.html.
[94] Bradlow RCJ, Berk M, Kalivas PW, Back SE, Kanaan RA. The Potential of N-Acetyl-L-Cysteine (NAC) in the Treatment of Psychiatric Disorders [published correction appears in CNS Drugs. 2022 Apr 28;:]. CNS Drugs. 2022;36(5):451-482. doi:10.1007/s40263-022-00907-3
[95] Bradlow RCJ, Berk M, Kalivas PW, Back SE, Kanaan RA. The Potential of N-Acetyl-L-Cysteine (NAC) in the Treatment of Psychiatric Disorders [published correction appears in CNS Drugs. 2022 May;36(5):553. doi: 10.1007/s40263-022-00925-1]. CNS Drugs. 2022;36(5):451-482. doi:10.1007/s40263-022-00907-3
[96] Morris G, Fernandes BS, Puri BK, et al. Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Aust N Z J Psychiatry. 2018 Oct;52(10):924-948. doi: 10.1177/0004867418796955. PMID: 30231628; Sarris J, Oliver G, Camfield DA, et al. N-Acetyl Cysteine (NAC) in the Treatment of Obsessive-Compulsive Disorder: A 16-Week, Double-Blind, Randomised, Placebo-Controlled Study. CNS Drugs. 2015 Sep;29(9):801-9. doi: 10.1007/s40263-015-0272-9. PMID: 26374743; Pittenger C. Pharmacotherapeutic Strategies and New Targets in OCD. Curr Top Behav Neurosci. 2021;49:331-384. doi:10.1007/7854_2020_204
[97] Riise GC, Qvarfordt I, Larsson S, et al. Inhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration. 2000;67(5):552-8. doi: 10.1159/000067473. PMID: 11070462
[98] Jakaria M, Azam S, Haque ME, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. doi:10.1016/j.redox.2019.101223
[99] Jakaria M, Azam S, Haque ME, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. doi:10.1016/j.redox.2019.101223
[100] Taleb S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front Immunol. 2019 Sep 10;10:2113. doi: 10.3389/fimmu.2019.02113. PMID: 31552046; PMCID: PMC6746884.
[101] Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998 Aug;3(4):271-80. PMID: 9727088.
[102] Forouzanfar F, Bazzaz BS, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17(12):929-938.
[103] Tiwari G, Gupta M, Devhare LD, Tiwari R. Therapeutic and Phytochemical Properties of Thymoquinone Derived from Nigella Sativa. Curr Drug Res Rev. Published online August 11, 2023. doi:10.2174/2589977515666230811092410
[104] Badary OA, Hamza MS, Tikamdas R. Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure. Drug Des Devel Ther. 2021;15:1819-1833. Published 2021 May 3. doi:10.2147/DDDT.S308863
[105] Arzanlou M. Inhibition of streptococcal pyrogenic exotoxin B using allicin from garlic. Microb Pathog. 2016 Apr;93:166-71. doi: 10.1016/j.micpath.2016.02.010. Epub 2016 Feb 18. PMID: 26911644; Cutler RR, Odent M, Hajj-Ahmad H, et al. In vitro activity of an aqueous allicin extract and a novel allicin topical gel formulation against Lancefield group B streptococci. J Antimicrob Chemother. 2009 Jan;63(1):151-4. doi: 10.1093/jac/dkn457. Epub 2008 Nov 11. PMID: 19001449.
[106] Torres KAM, Lima SMRR, Torres LMB, et al. Garlic: An Alternative Treatment for Group B Streptococcus. Microbiol Spectr. 2021 Dec 22;9(3):e0017021. doi: 10.1128/Spectrum.00170-21. Epub 2021 Nov 24. PMID: 34817207; PMCID: PMC8612145; Reiter J, Levina N, van der Linden M, et al. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules. 2017;22(10):1711. Published 2017 Oct 12. doi:10.3390/molecules22101711
[107] Zhang L, Wu X, Yang R, et al. Effects of Berberine on the Gastrointestinal Microbiota. Front Cell Infect Microbiol. 2021;10:588517. Published 2021 Feb 19. doi:10.3389/fcimb.2020.588517
[108]Sun D, Courtney HS, Beachey EH. Berberine sulfate blocks adherence of Streptococcus pyogenes to epithelial cells, fibronectin, and hexadecane. Antimicrob Agents Chemother. 1988;32(9):1370-1374. doi:10.1128/AAC.32.9.1370
[109] Du GF, Le YJ, Sun X, et al. Proteomic investigation into the action mechanism of berberine against Streptococcus pyogenes. J Proteomics. 2020 Mar 20;215:103666. doi: 10.1016/j.jprot.2020.103666. Epub 2020 Jan 23. PMID: 31981716; Henningham A, Döhrmann S, Nizet V, et al. Mechanisms of group A Streptococcus resistance to reactive oxygen species. FEMS Microbiol Rev. 2015 Jul;39(4):488-508. doi: 10.1093/femsre/fuu009. Epub 2015 Feb 10. PMID: 25670736; PMCID: PMC4487405.
[110] Dixit PV, Parihar G, Jain DK, et al. Increased serotonergic neurotransmission is not responsible for the anticompulsive effect of berberine in a murine model of obsessive-compulsive disorder. Behav Pharmacol. 2012 Oct;23(7):716-21. doi: 10.1097/FBP.0b013e328358477c. PMID: 22890212.
[111] Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159
[112] Szegedi A, Kohnen R, Dienel A, Kieser M. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine [published correction appears in BMJ. 2005 Apr 2;330(7494):759. Dosage error in article text]. BMJ. 2005;330(7490):503. doi:10.1136/bmj.38356.655266.82; De Vry J, Maurel S, Schreiber R, de Beun R, Jentzsch KR. Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur Neuropsychopharmacol. 1999;9(6):461-468. doi:10.1016/s0924-977x(99)00005-x
[113] Szegedi A, Kohnen R, Dienel A, et al. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): randomised controlled double blind non-inferiority trial versus paroxetine [published correction appears in BMJ. 2005 Apr 2;330(7494):759. Dosage error in article text]. BMJ. 2005;330(7490):503. doi:10.1136/bmj.38356.655266.82; Lyles JT, Kim A, Nelson K, et al. The Chemical and Antibacterial Evaluation of St. John’s Wort Oil Macerates Used in Kosovar Traditional Medicine. Front Microbiol. 2017;8:1639. Published 2017 Sep 8. doi:10.3389/fmicb.2017.01639
[114] Nicolussi S, Drewe J, Butterweck V, Meyer Zu Schwabedissen HE. Clinical relevance of St. John’s wort drug interactions revisited. Br J Pharmacol. 2020;177(6):1212-1226. doi:10.1111/bph.14936
[115] Ferko N, Levine MA. Evaluation of the association between St. John’s wort and elevated thyroid-stimulating hormone. Pharmacotherapy. 2001;21(12):1574-1578. doi:10.1592/phco.21.20.1574.34483
[116] Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. 2014;6:15-20. Published 2014 Feb 13. doi:10.2147/DHPS.S59665
[117] Sayyah M, Boostani H, Pakseresht S, Malayeri A. Comparison of Silybum marianum (L.) Gaertn. with fluoxetine in the treatment of Obsessive-Compulsive Disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):362-365. doi:10.1016/j.pnpbp.2009.12.016
[118] Offor SJ, Orish CN, Frazzoli C, Orisakwe OE. Augmenting Clinical Interventions in Psychiatric Disorders: Systematic Review and Update on Nutrition. Front Psychiatry. 2021;12:565583. Published 2021 May 5. doi:10.3389/fpsyt.2021.565583
[119] Lee B, Lee H. Systemic Administration of Curcumin Affect Anxiety-Related Behaviors in a Rat Model of Posttraumatic Stress Disorder via Activation of Serotonergic Systems. Evid Based Complement Alternat Med. 2018;2018:9041309. Published 2018 Jun 19. doi:10.1155/2018/9041309; Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159; Lopresti AL. Curcumin for neuropsychiatric disorders: a review of in vitro, animal and human studies. J Psychopharmacol. 2017 Mar;31(3):287-302. doi: 10.1177/0269881116686883. Epub 2017 Jan 30. PMID: 28135888.
[120] Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881-886. doi:10.2310/JIM.0b013e31821b8755
[121] Kuygun Karcı C, Gül Celik G. Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. Gen Psychiatr. 2020;33(2):e100159. Published 2020 Mar 11. doi:10.1136/gpsych-2019-100159

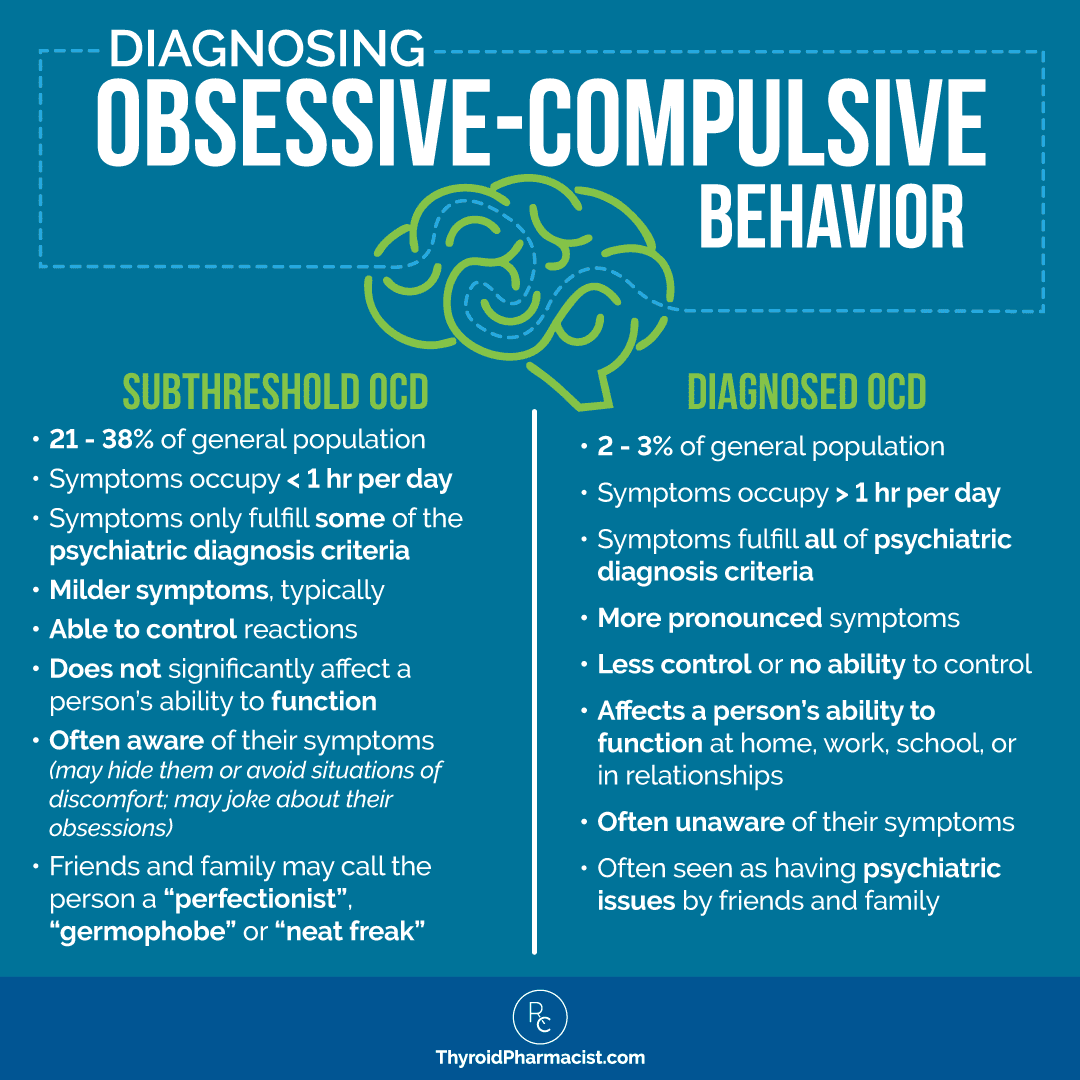
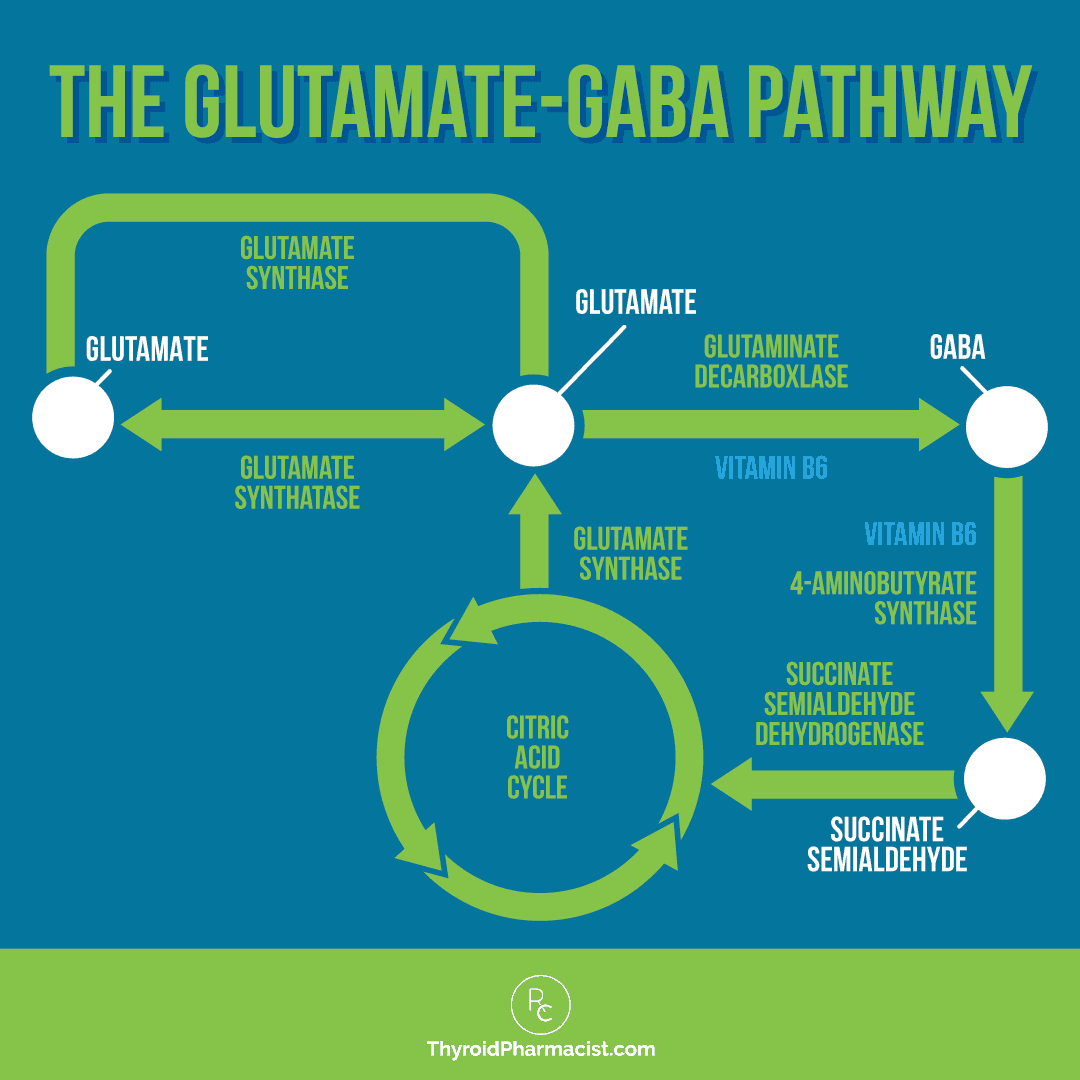

 Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
OMG! I have learned so much from your articles! As a child I had OCD tendencies and still get teased by my sibling for it. Knowing it might have been related to Hashimotos is both a relief and a let down. I can say, “So THAT might be why I was such a clean freak as a kid” but sad that my Hashimoto’s was not discovered or treated until I was a very tired mother of two. But going forward at least I can start to feel well now. Thank you!
Jane, thank you for sharing. I’m so glad to hear you found my article helpful.
thank you for this article. at 7 i had undiagnosed strep that turned into psoriasis. that cleared up and i was fine for awhile. i always had a tiny ocd that my Mom always made fun of me for. 10 years ago i started to get symptoms. neck and back pain, joint pain, my knees were the first to go when was still a kid, then fingers in high school. i always kind of assumed that was psoriatic arthritis. also horrible panic attacks at work. i went to doctors and nothing helped. then 5 years ago i started to have horrible fatigue that never went away. my anxiety and depression kept getting worse. my ocd was insane during covid. i went to doctors again and no one was able to pinpoint the problems. a year ago i developed blepharitis. my skin has been dryer than ever. my ocd is the worst it’s ever been. i started going to doctors again. i got blood work back late last night that screams hashimoto’s. everything makes sense now. the last year has been hell. but it all makes sense now.
Amanda, Thank you so much for sharing your story. I’m truly sorry to hear about everything you’ve been through, it sounds like it’s been a long and difficult journey. I know how frustrating it can be to go years without answers, and I’m so glad you’re finally getting some clarity. Hashimoto’s can affect so many different systems in the body, and once you have the right information, healing becomes so much more possible. Sending you love and encouragement as you move forward—you’re not alone. 💜