With the popularity of genetic and ancestry testing services such as 23andme, many people are now attempting to get a definitive “yes” or “no” answer as to whether they will get a given disease such as Alzheimer’s disease or even cancer.
Many of my clients and readers ask me if there are particular genes responsible for causing Hashimoto’s. I’ve had some readers even say that they believe their Hashimoto’s is “just genetic”; therefore, there’s nothing that can be done. Others are concerned that their family members may be affected…
They also ask if their children and other relatives are at greater risk, and whether genetic testing is the best way to know for sure.
While scientists are digging deeper and deeper into the genome and information becomes more readily available about things that influence gene expression, I want to provide some clarity in this department.
In the following article, you’ll learn:
- Is there a Hashimoto’s gene?
- What are the genes associated with thyroid disease?
- Do your genes sentence you and your loved ones to Hashimoto’s?
- How does our environment influence our genes?
- What you CAN change to reduce your risk of developing Hashimoto’s
- How to assess personal and family risk of Hashimoto’s
- Next steps if you find you have genes associated with thyroid disorders
- And more!
The Million Dollar Question… Is There a Single Hashimoto’s Gene?
No! There isn’t a single gene that has been identified as causing Hashimoto’s. Instead, there are a number of genes that have been “associated” with someone having a Hashimoto’s diagnosis.
Associated means that while the genes have been identified, they have not been proven to be the singular cause of the disease. When referring to these genes, I like to use the term “susceptibility genes”, as various combinations of them likely make an individual more susceptible to developing Hashimoto’s.
To further muddy the waters, we know that most genes – including the ones that have been seen in people with Hashimoto’s – can be “expressed” differently in different people.
This means that even if you have one or more of the susceptibility genes, that won’t necessarily mean you will be diagnosed with Hashimoto’s. The same goes for close family members; while they may share in one or more of these genes, whether or not they are at an increased risk will depend on how each of these genes is uniquely expressed.
New genes associated with Hashimoto’s are also still being identified in research, creating new influences on susceptibility, so there are lots of moving parts when it comes to the question of genetics.
Genes Play a Role, but Genes are Not Our Destiny
Genetics definitely play a role in the development of Hashimoto’s.
But to better explain how genes are just part of the puzzle, let me tell you about a study involving identical twins.
Identical twins share the exact same DNA. In conditions that are purely genetic, we will see that if one twin has a condition, then the other twin will have the same condition, 100% percent of the time. Yet in studies of twins where one twin has been diagnosed with Hashimoto’s, the other twin has only a 55 percent chance of developing it (as measured with thyroid antibodies).
So, how is that possible if they share the exact same DNA?
It is because of epigenetics, where genetic expression of one or more genes can be modified by “the environment”. Environmental influences consist of any number of non-genetic factors such as diet, infections, stress, toxin exposure, medications, etc. In the study above, the twins experienced differences in either their lifestyle or environment that influenced the expression of their genes. Perhaps there was an infection that kick-started the disease that only one experienced.
This is so exciting, as we can control many aspects of our lifestyle and environment!
Epigenetics means that your genes are not your destiny in most cases.
You can impact how genes are expressed, either resulting in more positive health outcomes, or worse.
You May Think, I’m Still Confused, Does Hashimoto’s “Run in Families” or Not?
Yes, there is definitely a genetic component. Research shows a higher incidence of the disease in families. Susceptibility genes can be passed on, especially to our children and first-degree relatives. In the twin study, there clearly was a greater incidence of Hashimoto’s in twins of people with Hashimoto’s than in the general population.
We know from the research that there are 3 requirements that must be present for an autoimmune disease to develop or be sustained. And genetic predisposition (or susceptibility) is only part of “the 3 legged stool” of developing autoimmune diseases such as Hashimoto’s.
Those 3 legs are:
- Genetic predisposition
- A trigger (this is that “environmental” factor – or factors – that impact how that genetic predisposition may be expressed)
- Intestinal permeability
Based on my clinical practice, if I had to apply a percentage as to the potential impact of each leg of the stool, I would say that genetic predisposition is only about 25 percent of the risk for developing Hashimoto’s.
That means that you have the power to change about 75 percent of the risk for getting Hashimoto’s.
The Power of the 75 Percent
Studies show that the rates of Hashimoto’s are going up with each passing year.
If you believe that genes can be turned on and off by environmental influences, maybe we need to look no further than the increasing environmental toxicity in our water, air, homes, and – ever increasingly – in our foods. Could this be the likely culprit? Creating both the trigger that impacts our epigenetics (to turn on the group of susceptibility genes that cause disease) as well as the intestinal permeability (leaky gut) further weakens our internal immune system and resilience.
In my research, I have found reason to believe that most of us actually have the genetic predisposition to develop autoimmune thyroid disease when presented with the right combination of triggers that overwhelm our body’s natural defenses.
A good example of this may be research done in 1997 relating to children who were young (birth through 7 years of age) at the time of the Chernobyl nuclear fallout, and who lived nearby. Researchers found that 80 percent of these children had thyroid antibodies, compared to just 17 percent of genetically similar children who lived farther away.
Clearly, the environment overwhelmed the bodies of those children living nearest to the fallout.
This type of research has led me to developing a theory relating autoimmune thyroid disease, which I call the Izabella Wentz Safety Theory.
This theory is based on adaptive physiology, along with other research, my work and observations working with Hashimoto’s. Adaptive physiology suggests that humans survive changes in their environment by developing chronic illnesses that actually are protective.
As an example, think about cavemen, and perhaps a time of famine due to wintry, inhospitable weather. Such a situation would signal to their bodies that it was not the best time to be fertile or to expend a lot of energy chasing hard to find food; instead, perhaps it was safest to conserve energy while awaiting the weather to change. So the cavemen’s bodies may have developed a protective chronic disease, something like Hashimoto’s, to slow down their metabolism and conserve their resources.
Thinking again about Chernobyl, we saw a lot of children within a specific vicinity and 80 percent ended up with Hashimoto’s antibodies. Why such a tremendous difference with other children outside that geography with similar genetic backgrounds? Could it be that these children’s bodies were sensing that the environment was dangerous, and triggering their autoimmune disorder as a protective measure, similar to the cavemen example?
In my clinical practice and working with a large number of people with Hashimoto’s, I have found that nutrient depletions, infections, an impaired stress response, an impaired ability to get rid of toxins, and many more non-genetic factors can trigger Hashimoto’s. All of these send a message to our body that the world is currently not a safe place and that the body should go into an energy conservation mode.
The takeaway here is that we may all have a genetic predisposition of some kind, for autoimmune diseases. Some, due to various gene combinations, may have greater susceptibility than others, but we may all have some degree of susceptibility. Sure, we’ve uncovered various genes that are associated with those having Hashimoto’s, but that doesn’t mean we won’t find more genes. And remember, epigenetics can turn all of those genes on and off.
So we need to be focusing on that other 75 percent of our risk! Again, the good news is that we can definitely modify our environment, hopefully reducing our triggers and/or intestinal permeability as well.
If so much of Hashimoto’s has to do with lifestyle decisions and environmental factors, should my relatives even worry about getting tested if they aren’t showing symptoms?
Yes!
And here’s why.
Genetics is still part of that 3-legged stool for developing autoimmune disease. If you have Hashimoto’s, you want to be proactive in establishing that other relatives don’t have it. They need to be aware that they, too, can take control of their health, ensuring that they do the right things to influence that 75 percent!
And we know that during the early stages of Hashimoto’s disease progression, that people do not test abnormal for TSH levels yet, and also do not likely exhibit thyroid-specific symptoms.
If you’ve been diagnosed with Hashimoto’s, think about the loved ones in your life like your mom, sister, daughter or aunt who may be at risk as well. Do you have women in your life that struggle with fatigue, depression, infertility, hair loss, cold intolerance, weight gain or anxiety?
While Hashimoto’s is much more common in women, (7 women are affected for every 1 man), men can also be affected.
In fact, if you are a man with Hashimoto’s, your relatives have a greater risk for the disease, and your children may also be at an increased risk.
What’s More Important to Test, Genes or Antibodies?
This is such a good question.
While there are a number of genes (about 20) that have been found to be associated with Hashimoto’s, getting your DNA tested – and interpreted – can be daunting for many people. And genes by themselves, as we’ve seen, may or may not result in Hashimoto’s.
Thyroid antibody testing, on the other hand, is easy to do… and the results are easy to understand. The presence of thyroid antibodies is really the best early warning system for knowing you are actively developing Hashimoto’s.
Thyroid antibodies can be elevated for 5 to 15 years before an abnormal TSH is even detected! And remember that elevated antibodies – even with a normal TSH – means that active destruction of the thyroid is already taking place.
Many clients come to me having been told their TSH is normal, but they still feel terrible. I struggled for almost a decade with progressively worsening symptoms including chronic fatigue, depression, anxiety, hair loss and many others. And throughout this time, my TSH was viewed as normal. By the time I was finally tested for thyroid antibodies, they were in the 2000 IU/mL range (optimal is less than 2 IU/mL!), and I was basically crippled by my poor health.
Had I had my antibodies tested earlier (and understood what they meant), I could have saved myself many years of misery.
Many of my clients are so relieved when they can provide this simple testing recommendation to close family.
For you to better understand why testing for thyroid antibodies can be such a great indication of your own risk of developing hypothyroidism, let’s discuss the first two stages that occur in Hashimoto’s as the disease develops. There are five stages in all.
In the initial stage, an individual has no symptoms. They have a normal TSH, normal thyroid function and will not have any elevated thyroid antibodies. All they have at this point is a genetic predisposition due to one or more of the susceptibility genes.
Keep in mind though, that some 75 percent of the risk is still affecting how those genes will be expressed.
Remember the 3 requirements (the 3-legged stool) for the disease to progress: genetic predisposition, one or more triggers, and intestinal permeability. These elements are going to impact the next stage of disease development. For some with the genes, no disease may occur. For others, they will start having thyroid antibodies in their system as the disease progresses.
So in stage 2, people start producing thyroid antibodies, but will still not test abnormal for TSH. They may exhibit a variety of non-specific symptoms such as weight gain, fatigue, anxiety, or just not feeling well. They may go on this way for years… remember my TSH tested normal for a decade before I finally had my antibodies tested.
If you have Hashimoto’s, wouldn’t you want to alert your relatives to the fact that they should be tested for antibodies (whether or not they have symptoms)? And note that some people may have depression and anxiety symptoms that they may be treating with prescriptions and therapy. They could be early-on in thyroid disease progression, and need interventions for Hashimoto’s instead of psychiatric care. (Read more about how Hashimoto’s can cause depression and anxiety.)
Many people have elevated thyroid antibodies for years (like I said, as many as 15 years!) before their TSH becomes elevated (considered stage 4 in the progression of Hashimoto’s disease).
I’ve written a two-part, very extensive article, on thyroid antibodies. Part one discusses more about why antibody testing is important, what antibodies you need to test for, what tests you can take, and a lot of other very helpful information. Part two talks about how you can reduce your antibodies through various interventions.
Antibody Prevalence in Families
There have been many inheritance studies done relating to the presence of thyroid antibodies in siblings and other family members of those with autoimmune diseases, including Hashimoto’s.
In fact, right after the discovery of thyroid antibodies, their presence was reported in 56 percent of siblings of those with autoimmune thyroid disease.
But before you say, “I thought genetics contributed to only 25 percent of the risk, so why such a high preponderance of the disease in siblings?”, let me comment on that.
Genetics play an important role because it is 1 leg of the 3-legged stool. And family studies – and studies done with first-degree relatives – show that genetic factors contribute to disease development.
But remember, the other 2 legs of the stool must be present for disease progression. And siblings and your children may share more than just one or more thyroid-related genes.
They probably share in environmental influences as well. They will likely eat the same diet, and potentially experience the same toxin exposures, perhaps even other Hashimoto’s triggers such as bacterial infections, a stressful environment, etc.
Familial clustering of Hashimoto’s has been seen in studies using the presence of thyroid antibodies as the measure. In one study, up to 50 percent of siblings of patients with Hashimoto’s were thyroid antibody positive in contrast to about 15 percent in the general population.
When both parents were affected, the prevalence of thyroid antibodies was 42 percent in daughters and 33 percent in sons, compared with 28.9 percent and 16.7 percent, respectively, when only one parent was positive for antibodies. Among first-degree relatives of children with Hashimoto’s, 34 percent had positive antibodies compared to only 13 percent of first-degree relatives of children without autoimmune thyroid disease.
So if you or a family member test positive for thyroid antibodies – even if your TSH levels are normal – you should have other close family members tested. Remember, antibodies can present themselves years – even a decade – prior to TSH levels testing as abnormal. Active thyroid damage is occurring during this time.
The Genes Associated with Thyroid Disease
The Human Genome Project has estimated that humans have between 20,000 and 25,000 genes. Most genes are the same in all people and everyone has two copies of each gene, one inherited from mom, and one from dad.
A small number of genes (less than 1 percent of the total) are slightly different between people. These are called alleles. Alleles are forms of the same gene with small differences. It is these differences that contribute to an individual’s unique physical features.
So for a gene that determines hair type, everyone has the gene for that. But some people may have the wavy allele and others, the straight hair allele. This gets more complicated when we talk about dominant and recessive alleles, in terms of what one will inherit and what we see run in families.
There have been more than 20 genes found that are associated with the clinical finding of autoimmune thyroid diseases, including both Hashimoto’s and Grave’s disease. Again, note that the term is “associated” versus “linked or causal” as none of these genes have been found to contribute more than a 4-fold increase in risk of developing one of these diseases. Association studies mean that genes contribute much smaller effects on disease susceptibility than through linkages.
For this reason, I like the term “susceptibility gene” as I feel it best depicts what these identified genes are. They are not the cause of Hashimoto’s or Graves’ disease. Variations in these genes make someone more susceptible. You also need particular variations to occur in a combination of genes (versus a singular gene). Researchers have found that there are also different combinations of these genes that can combine to cause the same clinical disease pattern.
The genes that have been found related to Hashimoto’s susceptibility can be roughly categorized as either immune regulatory genes or thyroid-specific genes. I will talk about a few of the genes that are believed to carry the highest risks for autoimmune thyroid diseases, as well as a few you may come across in your own genetics research.
First, I want to talk about polymorphisms.
Polymorphisms are variations that occur within a gene or in a regulatory region near a gene, and they play an important role in the expression of a given gene by affecting the gene’s function. When you see a gene listed below, know that there are one or more polymorphisms that are contributing to disease susceptibility, either within the given gene, or more often, outside of a given gene but nearby (and tied to the given gene).
Here are a few of the genes you may read about should you do genetic testing or research (and I’ll talk more about polymorphisms in a bit):
Immune regulatory genes associated with autoimmune thyroid diseases include:
- HLA complex genes
- CTLA4
- PTPN22
- CD40
- CD25
- FCRL3
- FOXP3
- SH2BC
The thyroid-specific genes associated with autoimmune thyroid diseases include:
- TG
- TSHR
- CAPZB
- PDE8B
- FOXE1
- VAV3
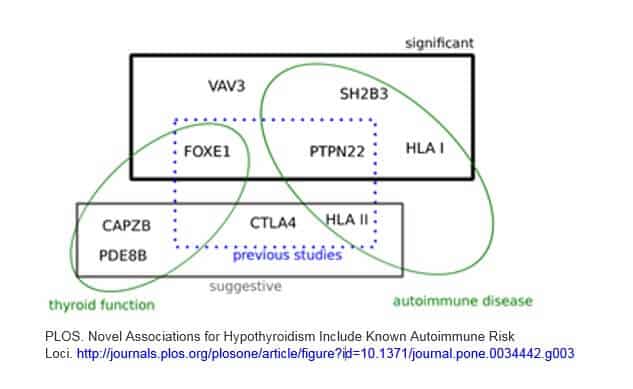
I found a good graphic in a study that I am including here. While it doesn’t cover all of the above genes, I think it is a helpful visual to illustrate a few points.
1) There are a combination of gene variants that are involved
2) Autoimmune genes play a significant role in Hashimoto’s (many of these same genes are also found in a variety of other autoimmune disorders)
3) Genes that are related to thyroid function and hormone levels are part of the puzzle
4) There are new, emerging genes being found… on top of previous studies
This graphic is from a 2012 study which contains a good overview on this topic. Although it is quite technical, you can access it here.
Polymorphisms (When the Gene is Expressed for Disease)
Of course, it isn’t as easy as looking to see if you have these susceptibility genes, as again, we all do! It is how they are expressed and what variations – or mutations – they may have undergone that counts.
Polymorphisms are the most common type of genetic variation among people. The term you have likely run across is SNPs. This is pronounced “snips”, and refers to single nucleotide polymorphisms.
It is the polymorphisms related to the above genes that likely contribute to the predisposition for Grave’s disease, Hashimoto’s and even other autoimmune diseases.
Each SNP represents a variation or difference in a single DNA building block, called a nucleotide. There are a lot of possible SNPs, some 10 million in the human genome!
Many SNPs don’t impact a person’s health. But many do, and they can do so in a variety of ways. Scientists have found SNPs that may help predict a person’s susceptibility to environmental toxins, or how they will respond to a particular drug, as well as their risk for developing various diseases.
Scientists think that over 90 percent of genetic risk factors are actually found outside the actual gene. They believe it is the SNPs in the vicinity of identified genes that influence one or more gene’s expression.
SNPs are depicted using a number and are either related to or contained within a gene. So for the gene, CTLA4, noted above, as one example, two SNPs are associated with Graves’ disease susceptibility. These two SNPs are denoted as: rs231775 and rs733618. The same gene also has a SNP that is associated with Hashimoto’s, rs231779. And if you look on a site such as LiveWello, you’ll see that this gene, CTLA4, also has many more SNPs that are associated with many other autoimmune diseases including Celiac disease, Lupus and Diabetes.
You can see how complicated determining your genetic risk can be. It isn’t just a matter of your having a combination of given genes, but you also need to have particular SNPs that have been shown to influence one or more of the given genes.
If you are going to try and figure out your genetic disposition, or that of family members, through genetic testing, a test like 23andMe will give you a data dump of SNP info, entirely too much to wade through manually. You’ll need to use another program, such as NutraHacker, LiveWello or Genetic Genie, to take your raw data and interpret it for you given the magnitude of SNPs you will have.
Ethnicity also comes into play relating to what the research has found for genes and SNPs, with some SNPs only influencing certain ethnicities (as shown in research). It can get quite complicated and will involve a lot of dedication from you to really understand your 23andMe report should you take your data to the next level. But doing this type of biohacking can also be fascinating and rewarding.
One final comment about polymorphisms and the genetics of Hashimoto’s is that there is a definite impact to the expression of genes depending on whether it is a maternal or paternal X chromosome. We know women are diagnosed with Hashimoto’s more often than men. There are a number of theories about this, and scientists are still not sure as to why that is the case. You can read more about women having a preponderance of thyroid disease, and why that might be so, here.
Here are some of the commonly known genes that have been implicated as susceptibility genes for Hashimoto’s, Grave’s disease and autoimmune diseases in general. Remember, they all have one or more SNPs associated with them.
Immune Regulatory Genes
The majority of the genes identified to be a factor in autoimmune diseases are immune regulatory genes involved in ensuring immune responses against foreign antigens while maintaining what is called “tolerance”, to self-antigens. If these genes are influenced in a negative way by SNPs, this can lead to a problem in the body’s immune tolerance, which would ultimately result in autoimmunity of some kind.
HLA Complex (HLA-DR3)
The Human Leukocyte Antigens family of genes is often referred to as the HLA complex. The HLA complex helps the body’s immune system distinguish its own proteins from proteins made by foreign invaders such as bacteria and viruses.
It makes such sense that this family of genes would be highly associated with an autoimmune condition such as Hashimoto’s!
The HLA-DRB1 gene variant is one variant involved in this immune regulatory process. But it is the HLA-DR3 gene variant that appears to carry the highest risk (seen in Grave’s disease and also in Hashimoto’s).
Just to complicate things even further, note that not every patient with Graves’ disease has the HLA-DR3 gene. You can see there are a variety of susceptibility genes for different diseases, and scientists likely haven’t yet uncovered them all.
There is a HLA-DR5 gene associated with having an enlarged thyroid (goitrous form).
More than 100 diseases have been associated with different alleles of HLA genes. Many other disorders involving abnormal immune function have also been associated with specific HLA alleles.
CTLA4
As already discussed, SNPs linked to this gene have been associated with a wide variety of autoimmune diseases. This is referred to as being a highly polymorphic gene.
The CTLA4 gene transmits an inhibitory signal to T cells, and it is often found in association with other genes common to autoimmune disorders, such as PTPN22, CD40, CD25 and FCRL3. The CTLA-4 gene was reported to be linked and/or associated with type 1 diabetes mellitus, asthma, Addison’s disease, myasthenia gravis, systemic lupus erythematosus, ulcerative colitis, and with all forms of autoimmune thyroid disease.
Several of the genes associated with Hashimoto’s, like the CTLA4 gene, are vital to T cell regulation, which plays an important role in preventing autoimmunity.
CTLA4 may also play a role in the susceptibility to high levels of antibodies.
PTPN22
This is another gene that is involved with T cell regulation as a powerful inhibitor of T cell activation, and has been associated with Grave’s Disease and Hashimoto’s.
About 65 percent of the general population carries an allele (the A allele) of a certain SNP (rs4704397) which increases the risk for hypothyroidism.
Additional studies have found another SNP (R620W) of the PTPN22 gene that is associated with other autoimmune diseases, including type 1 diabetes mellitus.
CD40
This gene is specific to Grave’s disease, with several SNPs within the gene identified as predisposing to Grave’s Disease. CD40 SNPs have also been found in a wide range of autoimmune and immune-mediated diseases including lupus, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis.
CD25
This is another gene that is involved with T cell regulation, but has only been associated with Grave’s disease. Variants in this gene can cause lower or higher levels of suppressor T cells. Higher levels can lead to impairment of the body’s ability to effectively respond to infectious invaders. Lower levels can result in a variety of immune-related conditions.
SNPs of the gene have been associated with multiple sclerosis and Type 1 diabetes, as well as Grave’s disease.
FCRL3
Genetic variation in this gene may influence susceptibility to a wide variety of autoimmune disorders, including rheumatoid arthritis, autoimmune thyroid disease (both Grave’s disease and Hashimoto’s), multiple sclerosis and systemic lupus erythematosus.
FOXP3
Various FOXP3 SNPs have been reported to be associated with both Grave’s disease and Hashimoto’s. This gene is involved in the normal function of regulatory T cells. The protein that the gene produces, the FOXP3 protein, is found primarily in the immune system gland called the thymus (where regulatory T cells are produced).
SH2B3
One SNP of this gene, rs3184504, is associated with a number of autoimmune conditions. The C version of this SNP is associated with slightly lower odds of hypothyroidism.
Thyroid Specific Genes
There are a handful of genes that are not immune regulatory but are actual thyroid specific genes. Identified thyroid-specific genes include TG and TSHR, but not TPO. A possible explanation of this is that TPO is not polymorphic.
TG
The TG gene provides instructions for making a protein found only in the thyroid gland, called thyroglobulin. Thyroglobulin combines with iodine, breaking down to release thyroid hormones. A SNP for TG was identified as a variant that predisposes to autoimmune thyroid disease.
TSHR
This gene is tied primarily to Grave’s disease, although TSHR gene mutations have been identified in people with congenital hypothyroidism (abnormally low levels of TSH hormones starting from birth).
CAPZB
This gene has a nearby SNP associated with TSH levels, as well as levothyroxine responsiveness.
PDE8B
This gene also has an SNP associated with TSH levels.
FOXE1
SNPs correlated with this gene have been associated with both TSH levels as well as thyroid cancer. FOXE1 is also involved in thyroid development, in particular, mutations causing congenital hypothyroidism. Variants near FOXE1 were identified as genetic risk factors for primary hypothyroidism as well.
VAV3
One SNP of this gene, rs12126655, was found to be associated with TSH concentrations.
Can Other Genes Affect my Having Hashimoto’s?
Yes! There are other genes that can contribute to the Hashimoto’s puzzle because there are genes that can potentially result in triggers; and remember, triggers are one leg of the 3-legged stool for having an autoimmune disease.
Triggers can be addressed through lifestyle interventions, so it can be helpful to know if you have a gene variant that is known to cause problems.
There are genes that affect nutrient absorption and detoxification, for example. A common gene variant that I see in my clients is the MTHFR gene, so let’s talk briefly about that.
Variants of this gene can cause methylation impairment. What is that? Methylation is one of the body’s core detox processes that helps your body rid itself of toxins (and remember, toxins can cause inflammation and also be triggers). Toxins can include heavy metals and other chemicals, and can also include excess hormones such as estrogen. Poor methylation can lead to many issues including estrogen dominance, which can be a trigger for Hashimoto’s. You can learn more about estrogen dominance here (watch my short video!).
There are two MTHFR SNPs that are commonly seen (the C677T gene and the A1298C gene), and although there isn’t research substantiating a greater prevalence of these in people having autoimmune disease, I believe that they are common in those with Hashimoto’s based on what I see with clients and readers.
I have this gene variation (C677T).
You can read much more about the MTHFR gene and its potential effect on people having Hashimoto’s, here. This article will also point to a simple test that can be done specific to testing for the MTHFR gene, as well as protocols I recommend to address nutrient deficiencies and detoxification impairments caused by the MTHFR gene variations.
Any type of testing, including genetics testing or specific testing for the MTHFR gene, can provide useful information that can help point you to steps that you can take to reduce your health risks.
I feel data is empowering, so I have always jumped on the opportunity to do testing of all kinds. But I know it can get expensive, so I encourage my clients to do testing where it can have the biggest impact to their health. Knowledge allows you to intervene and influence the direction your health will take. Remember, you have control of the 75 percent!
Genes are Not Your Destiny… You Can Influence the Risk!
Remember the twin studies, in which exact matches of genes resulted in two different health outcomes. One twin had Hashimoto’s and the other had no measurable thyroid antibodies. Clearly, there is more than genetics at play.
You can view your DNA as 100 percent of what your health will absolutely be; or you can view your DNA as how your health has simply been genetically primed, but can still be influenced one way or the other.
Perhaps you’ve done a 23andMe test, uploaded your data to a SNP processing site, and have found that you have a handful of SNPs associated with the genes noted earlier. Maybe you are more susceptible to thyroid autoimmune disorders, now what?
What Should You Do?
Remember that genes only make you more susceptible. So how do you know what’s really happening in your body?
First, get tested for thyroid antibodies, as it is an easy, inexpensive way to measure whether you are at risk. Should you already be diagnosed with Hashimoto’s, suggest to your siblings, children and close relatives that they, too, should get tested. Early identification of thyroid antibodies allows them to focus on what they can do to ensure they do not have the 3 legs of the 3-legged stool. Remember the 5 stages of Hashimoto’s development? If they test positive for antibodies, they are already in stage 2 and active damage is occurring to their thyroid.
Educate yourself on triggers and intestinal permeability. If you have the genetic predisposition, it is even more important to understand how various triggers (the second leg of the 3-legged stool) such as infections, diet or stress, can affect your health. It is also more important that you focus on protocols that can best support your not having intestinal permeability (the third leg of the 3-legged stool).
Reducing common triggers can make you feel better! In the summer of 2015, I developed a survey to assess the impact of various interventions on Hashimoto’s. Some 2,232 people with Hashimoto’s provided feedback as to the factors that seemed to make their conditions better as well as worse. Eighty-eight percent of people with Hashimoto’s who became gluten free felt better, and 33 percent had a reduction in their thyroid antibodies on a gluten free diet. And this is just one example. Read more about the results here.
Consider testing for the MTHFR gene variant that could be worsening your Hashimoto’s condition. In my survey, 45 percent of people with Hashimoto’s said they felt better after adding methylation supporting supplements to their regimen.
The more you know, the more you will feel empowered to influence the trajectory of your health.
When you sign up for my newsletter, you can also download a free chapter of my Root Cause book, recipes, and a Thyroid Diet Starter Guide. You will also receive occasional updates about new research, resources, giveaways and helpful information.
For future updates, make sure to follow me on Facebook!
References
- Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. Journal of Autoimmunity. 2008;30(1-2):58-62. doi:10.1016/j.jaut.2007.11.010.
- Vykhovanets E, Chernyshov V, Slukvin I et al. 131I Dose-dependent thyroid autoimmune disorders in children living around Chernobyl. Clinical Immunology and Immunopathology. 1997;84(3):251-259. doi:10.1006/clin.1997.4379.
- Zaletel K, Gaberšček S. Hashimoto’s thyroiditis: From genes to the disease. Current Genomics. 2011;12(8):576-588. doi:10.2174/138920211798120763.
- Davies T, Latif R, Yin X. New Genetic Insights from Autoimmune Thyroid Disease. Journal of Thyroid Research. 2012. doi:10.1155/2012/623852.
- Genetics Home Reference. What was the Human Genome Project and why has it been important?. NIH.
- https://ghr.nlm.nih.gov/primer/hgp/description. Updated March 27, 2018. Accessed March 20, 2018.
- Sgarbi JA. Autoimmune thyroid disease: What secrets we still need to unravel?. Archives of Endocrinology and Metabolism. 2015;59(2):95-97. https://dx.doi.org/10.1590/2359-3997000000019
- Jonkers IH, Wijmenga C. Context-specific effects of genetic variants associated with autoimmune disease. Human Molecular Genetics. 2017;26(R2):R185–R192. https://doi.org/10.1093/hmg/ddx254
- Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of Autoimmune Thyroid Diseases: A comprehensive review. Journal of autoimmunity. 2015;64:82-90. doi:10.1016/j.jaut.2015.07.009.
- Jacobson EM, Tomer Y. The CD40, CTLA-4, Thyroglobulin, TSH Receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. Journal of autoimmunity. 2007;28(2-3):85-98. doi:10.1016/j.jaut.2007.02.006.
- Tomer Y. Genetic susceptibility to autoimmune thyroid disease: Past, present, and future. Thyroid. 2010;20(7):715-725. doi:10.1089/thy.2010.1644.
- GB Health Watch. Iodine. GB Health Watch. https://www.gbhealthwatch.com/Nutrient-Iodine-Genes.php Published in 2018. Accessed March 20, 2018.
- Alcina A, Fedetz M, Ndagire, et al. IL2RA/CD25 gene polymorphisms: uneven association with multiple sclerosis (MS) and type 1 diabetes (T1D). Plos One. 2009. doi:10.1371/journal.pone.0004137.
- FCRL3 Fc receptor like 3 [ Homo sapiens (human) ]. NCBI. https://www.ncbi.nlm.nih.gov/gene/115352. Updated on March 29, 2018. Accessed March 20, 2018.
- Dechairo B, Zabaneh D, Collins J, et al. Association of the TSHR gene with Grave’s disease: the first disease specific locus. European Journal of Human Genetics. 2005;13:1223-1230. doi:10.1038/sj.ejhg.5201485.
- Eriksson N, Tung J, Kiefer A, et al. Novel associations for hypothyroidism include known autoimmune risk loci. Plos One. 2012. Doi:10.1371/journal.pone.0034442.
Genetics Home Reference. FOXE1 gene. NIH. https://ghr.nlm.nih.gov/gene/FOXE1#conditions. Updated on March 27, 2018. Accessed March 20, 2018. - Koc A, Sayitoglu M, Karakurt F, et al. Association of three SNPs in the PARP-1 gene with Hashimoto’s thyroiditis. Human Genome Variation. 2014;1(14016). doi:10.1038/hgv.2014.16.



 Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
Disclosure: As an Amazon Associate I earn from qualifying purchases. We are a professional review site that receives compensation from the companies whose products we review. We test each product thoroughly and give high marks to only the very best. We are independently owned and the opinions expressed here are our own.
wow so much to take in, but necessary. ty isabella.
Claire – thank you! I understand how overwhelming this can be. Developing autoimmunity is like a three-legged stool, (Genetic predisposition, Environmental triggers and Intestinal permeability (leaky gut) all of these factors must be present for autoimmunity to occur! When you remove one of these, you can prevent or stop autoimmune disease. While we can’t change genes, if we know the trigger, we can remove it and we can heal the gut. Here are some links which might help:
REVERSING AUTOIMMUNITY? AND THE PERFECT STORM
https://thyroidpharmacist.com/articles/reversing-autoimmunity-and-the-perfect-storm/
IS HASHIMOTO’S HYPOTHYROIDISM GENETIC?
https://thyroidpharmacist.com/articles/is-hashimotos-genetic
Thank you, thank you, thank you! Your ongoing research and shared findings are invaluable. You present critical information from current research discoveries published in research articles most of us wouldn’t otherwise have access to and you do it in a language we understand. With so much research data out there in recent years that supports the value of genetic testing in achieving improved clinical outcomes, it is unconscionable that only a limited percentage of physicians are ordering genetic testing for their patients, as well as, the high percentage of physicians that have refused or failed to act on their patient’s requests for genetic testing despite poor or very limited clinical treatment outcomes. One of the triggers of disease that physicians rarely recognize, suspect or consider as a possible cause of any disease and still remains elusive and under the medical community radar are medications. A significant number of the most widely prescribed medications used to treat one health condition have been later found to cause another. Part of the problem is that despite a significant number of patients reporting to their prescribing physician their health declined or failed to improve after prescribed treatment with a specific drug, their physicians had denied the medication had caused their symptoms or led to the development of another disease, even in those without a genetic predisposition or susceptibility to develop the disease. How many drug investigations had been initiated after physicians reported the drugs they prescribed had caused serious side effects and complications to their patient’s health? I suspect few to none. I believe most drug safety investigations had resulted after patients reported the drug themselves to a pharmaceutical watchdog organization that applied pressure on the FDA to conduct an investigation. Drugs known and proven to cause Diabetes in a large population of patients have now been identified and warnings have been reported to consumers. Some blood pressure drugs have been shown to cause acute leukemia, blindness and age-related-macula degeneration, but they are still manufactured and prescribed to unsuspecting patients. Some birth control pills have caused blood clots, and despite the numbers of reported deaths as early as the mid-1970s after blood clots formed in the hepatic arteries of young women, doctors still prescribed the same birth control pill. And of course, your thyroid pharmacist advocacy efforts and discovery that some thyroid medications contained wheat starch as a filler and the serious complications, the development of other autoimmune diseases and the poor response to treatment in many patients with a confirmed diagnosis of hypothyroidism had to be addressed and resolved. Because of you, your research, your dedication to empowering those suffering from thyroid disease through education and support, and your thyroid pharmacist advocacy for those that would otherwise remain unheard, thyroid disease sufferers are reclaiming their health and are overcoming a poor quality of life. From my perspective, you have saved lives that wouldn’t have otherwise been saved, and that in itself, is truly heroic!
found to cause are expanded
Judy – thank you so much for your kind words and support! <3 How is your thyroid healing journey going?
I didn’t find that genetic information was critical to finding a solution in my case. My reason is that, although I had non-celiac gluten intolerance (NCGI), the genes that are supposedly implicated in gluten problems (HLA-DQ2, HLA-DQ8) said that I did not have a vulnerability to gluten intolerance. I did consult with Ron Hoggan on the subject of NCGI and was told the condition was not (at that time) recognized by the medical community, and that research has shown that NCGI may be a problem in up to 12% of the population (as compared to 1% for celiac). I am more inclined to blame the problem on environmental factors, specifically, the known toxicity of dwarf wheat.
Wondering where to get a Gene’s test from? I cant see the main hashimotos snps in 23andme. I was recently tested. Have they taken this SNP out?
Also does DIO1 or DIO2 have the biggest impact on t3-t4 conversion. I see 23andme records only DIO1, but not DIO2?
Nicki – thank you for reaching out. I would recommend that you contact 23andme abot what snps they do or do not include in their testing. As for DIO1/DIO2 impact on T3-T4 conversion, unfortunately I don’t have any information to share with you at this time. I will be happy to add this to my list of future article possibilities.